��Ŀ����
����˵����ȷ����
A����֪0.1 mol��L-1�Ĵ�����Һ�д��ڵ���ƽ�⣺CH3COOH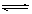 CH3COO����H�����������ռ���Һ��ʹ��Һ�� CH3COO����H�����������ռ���Һ��ʹ��Һ��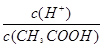 ֵ���� ֵ���� |
B��25��ʱ����ˮ�м�����������CH3COONa��ˮ�ĵ���ƽ�⣺H2O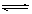 H++OH-�����ƶ���c(H+)���� H++OH-�����ƶ���c(H+)���� |
| C��ȡc(H+)="0.01" mol��L-1������ʹ����100 mL���ֱ�ϡ��2�����ٷֱ����0.03 gп�ۣ�����ͬ�����³�ַ�Ӧ��������п��Ӧ�����ʴ� |
| D������������Һ�еμ�ϡ����õ���pH��5�Ļ����Һ�У�c(Na��)��c(NO3��) |
C
���������
A��
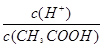 =
= ����������������Һ��c(CH3COO-)����
����������������Һ��c(CH3COO-)����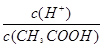 ��С������
��С������B��ˮ�м�������ƹ��壬CH3COO-��H+��ϣ�ˮ�ĵ���ƽ�������ƶ�������
C��ͬpH������ʹ���ϡ����ͬ�ı���������c(H+)����Zn��Ӧ���ʿ죬��ȷ��
D����������Һ�м������ᣬc(Na+)��c(NO3-)���䣬c(Na��)=c(NO3��)������

��ϰ��ϵ�д�
�����Ŀ
 H++SO42-��
H++SO42-�� H����HCO3�������� HCO3��
H����HCO3�������� HCO3��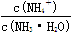 ������
������  NH4++OH�� ����������������ʱ���ı�����������ƽ�������ƶ�����NH4+Ũ��������ǣ� ��
NH4++OH�� ����������������ʱ���ı�����������ƽ�������ƶ�����NH4+Ũ��������ǣ� �� 2B��g������H��������Ӧ�Ļ��ΪEa kJ��mol-1���淴Ӧ�Ļ��ΪEb kJ��mol-1�����H =��Ea ��Eb��kJ��mol-1��
2B��g������H��������Ӧ�Ļ��ΪEa kJ��mol-1���淴Ӧ�Ļ��ΪEb kJ��mol-1�����H =��Ea ��Eb��kJ��mol-1��