��Ŀ����
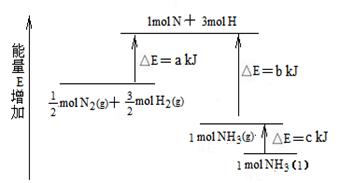
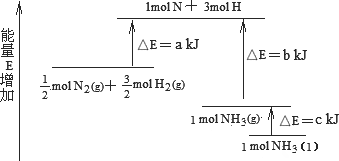
��ҵ�ϳɰ��ķ�ӦN2��3H2��2NH3�������仯����ͼ��ʾ����ش��й����⣺
(1)�ϳ�1 mol��NH3(l)________(����ա������ų���)________kJ��������
(2)��֪��
��1 mol��H��H����1 mol��N��H����1 mol��N��N ���ֱ���Ҫ��������436 kJ��391 kJ��946 kJ������ͼ�е�a��________kJ��1 mol��N2(g)��ȫ��Ӧ����NH3(g)�����������仯Ϊ________KJ��(3)�ƲⷴӦ2NH3(l)��N2(g)��3H2(g)�ȷ�Ӧ2NH3(g)��N2(g)��3H2(g)________(����ա������ų���)������________(��ࡱ�����١�)��
�𰸣�
������
������
|
����(1)�ų���b��c��a ����(2)1127��92 ����(3)���ա��� |

��ϰ��ϵ�д�
�����Ŀ
 2NH3��g���������ݻ�Ϊ2.0L���ܱ������г���0.60mol N2��g����1.60molH2��g������Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ���������NH3�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮�ȣ�Ϊ
2NH3��g���������ݻ�Ϊ2.0L���ܱ������г���0.60mol N2��g����1.60molH2��g������Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ���������NH3�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮�ȣ�Ϊ N2��g��+3H2��g����ƽ�ⳣ����
N2��g��+3H2��g����ƽ�ⳣ����