��Ŀ����
ij��ȤС����ʵ������ͭ������Ϊԭ�ϣ����ö��ַ�����ȡ����ͭ���Ʊ��������£�
����һ
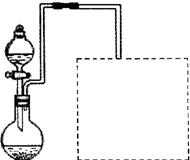
�ż�ͬѧȡ6.4 gͭƬ��10 mL 18 mol��L��1Ũ���ᣬ�����Թ��й���ʱ���֣�ͭ���ȵ�Ũ���ᷴӦ��û�еõ�Ԥ�ڵ���ɫ��Һ���������Թܵײ�������ɫ��������ͬѧΪ����֤���а�ɫ��������Ҫ�ɷ֣��������ʵ�顣
ʵ�鲽�裺�㵹���ϲ�Һ��������ð�ɫ�Ĺ����м�����������ˮ���ӱ߽��衣
ʵ������ɫ�����ܽ⣬��Һ��Ϊ��ɫ��
ʵ����ۣ����ð�ɫ����Ļ�ѧʽΪ ��
��2����ͬѧ���ͬѧ����ͬ��ʵ�飬���۲쵽���ȹ����У��Թ��ڱ��ϲ�������������ɫ�������ʣ��������ȣ�����ɫ��������������������Ũ�������ʧ��ͬʱ������ʹƷ����Һ��ɫ�����壬����ɫ������ʧ��ԭ����(�û�ѧ��Ӧ����ʽ�ش�) ��ֱ�����Ӧ��ϣ������Թ��л���ͭƬʣ�ࡣ
������
��3����ͬѧ��Ϊ����Ƶ�ʵ�鷽�����ã����Լ���Ƶ�˼·�ǣ�2Cu��O2 2CuO��CuO��H2SO4��CuSO4��H2O��
2CuO��CuO��H2SO4��CuSO4��H2O��
�Աȼķ���������Ϊ��ͬѧ���ŵ��Ǣ�_________________________����_ ��
������
�ȶ�ͬѧȡһͭƬ��ϡ��������Թ��У��������е���˫��ˮ��������Һ����ɫ��д����Ӧ�Ļ�ѧ��Ӧ����ʽ ��
(9��)[��1��CuSO4
(1��) ��2��S��2H2SO4(Ũ)  3SO2����2H2O (2��)
3SO2����2H2O (2��)
��3���ٲ�������������ͭ�����ĵ�������� (2��)���ڲ�������Ⱦ��SO2 (2��)
��H2SO4��Cu��H2O2��CuSO4��2H2O (2��)
��������
�����������1����ɫ�Ĺ����м�����������ˮ����ɫ�����ܽ⣬�ҹ۲쵽��ҺΪ��ɫ����˵�����ð�ɫ����ΪCuSO4��
��2���Թ��ڱ��ϲ�������������ɫ�������ʣ��ɴ��ڵ�Ԫ�ؿ�֪����ɫ��������ΪS��S���л�ԭ�ԣ�Ũ�������ǿ�����ԣ�S��Ũ���ᷢ��������ԭ��Ӧ������ʽΪS��2H2SO4(Ũ)  3SO2����2H2O���Ӷ�ʹ����ɫ������ʧ��
3SO2����2H2O���Ӷ�ʹ����ɫ������ʧ��
��3���ɷ�Ӧ����ʽ2Cu��O2 2CuO��CuO��H2SO4��CuSO4��H2O��֪���������ж������������������ŵ�Ϊ�������ж�������Ⱦ����������Ԫ��ȫ��ת��Ϊ����ͭ����˲�������������ͭ�����ĵ�������١�
2CuO��CuO��H2SO4��CuSO4��H2O��֪���������ж������������������ŵ�Ϊ�������ж�������Ⱦ����������Ԫ��ȫ��ת��Ϊ����ͭ����˲�������������ͭ�����ĵ�������١�
��4��˫��ˮ����ǿ�����ԣ�����������ͭ�����ͭƬ��ϡ��������Թ��У��������е���˫��ˮ��������Һ����ɫ���������ڷ�����������ԭ��Ӧ���µģ��÷�Ӧ��ѧ����ʽΪH2SO4��Cu��H2O2��CuSO4��2H2O��
���㣺����Ũ��������ʣ�ͭ����������Ҫ���������Ҫ���ʣ���ѧʵ�鷽������������۵�

 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д�