��Ŀ����
��2010?��������ģ����������ƣ�Na2S2O3�������������ƺ����ͨ�����Ϸ�Ӧ�Ƶã���֪��Na2S2O3��������Һ�в����ȶ����ڣ�
��1��ij�о�С��������Ʊ�Na2S2O3?5H2O��װ�úͲ��ֲ����������£�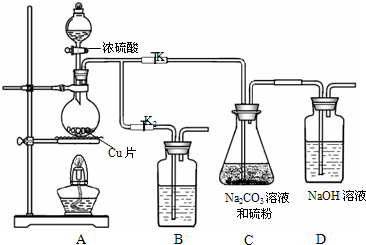
��K1���ر�K2����Բ����ƿ�м�������Ũ���ᣬ���ȣ�
��C�еĻ��Һ��������������Ӧһ��ʱ�����۵������٣���C����Һ��pH�ӽ�7ʱ��ֹͣC�еķ�Ӧ��ֹͣ���ȣ�
����C�еĻ��Һ��
��������Һ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ���ɣ��õ���Ʒ��
�٢��У�Բ����ƿ�з�����Ӧ�Ļ�ѧ����ʽ��
�ڢ��У�����C����Һ��pH�ӽ�7ʱ��ֹͣC�еķ�Ӧ����ԭ����
��ֹͣC�еķ�Ӧ���IJ�����
�ۢ��У������ˡ��õ��IJ��������ǣ����������ƣ�
��װ��B��ʢ�ŵ��Լ��ǣ��ѧʽ��
��2�����ݷ�Ӧ2S2O32-+I2=S4O62-+2I-������I2�ı���Һ�ⶨ��Ʒ�Ĵ��ȣ�ȡ5.5g ��Ʒ�����Ƴ�100mL��Һ��ȡ10mL��Һ���Ե�����ҺΪָʾ������Ũ��Ϊ0.050mol/L I2�ı���Һ���еζ���������ݼ�¼���±���ʾ��
���жϴﵽ�ζ��յ��������
��Na2S2O3?5H2O�ڲ�Ʒ�е����������ǣ�����������1λС����
��1��ij�о�С��������Ʊ�Na2S2O3?5H2O��װ�úͲ��ֲ����������£�

��K1���ر�K2����Բ����ƿ�м�������Ũ���ᣬ���ȣ�
��C�еĻ��Һ��������������Ӧһ��ʱ�����۵������٣���C����Һ��pH�ӽ�7ʱ��ֹͣC�еķ�Ӧ��ֹͣ���ȣ�
����C�еĻ��Һ��
��������Һ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ���ɣ��õ���Ʒ��
�٢��У�Բ����ƿ�з�����Ӧ�Ļ�ѧ����ʽ��
Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O
| ||
Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O
��
| ||
�ڢ��У�����C����Һ��pH�ӽ�7ʱ��ֹͣC�еķ�Ӧ����ԭ����
Na2S2O3��������Һ�в����ȶ�����
Na2S2O3��������Һ�в����ȶ�����
����ֹͣC�еķ�Ӧ���IJ�����
��K2���ر�K1
��K2���ر�K1
���ۢ��У������ˡ��õ��IJ��������ǣ����������ƣ�
©�������������ձ�
©�������������ձ�
����װ��B��ʢ�ŵ��Լ��ǣ��ѧʽ��
NaOH
NaOH
��Һ������������C�еķ�Ӧֹͣ������A�в����Ķ���SO2����ֹ������Ⱦ
��C�еķ�Ӧֹͣ������A�в����Ķ���SO2����ֹ������Ⱦ
����2�����ݷ�Ӧ2S2O32-+I2=S4O62-+2I-������I2�ı���Һ�ⶨ��Ʒ�Ĵ��ȣ�ȡ5.5g ��Ʒ�����Ƴ�100mL��Һ��ȡ10mL��Һ���Ե�����ҺΪָʾ������Ũ��Ϊ0.050mol/L I2�ı���Һ���еζ���������ݼ�¼���±���ʾ��
| �� �� | 1 | 2 | 3 | 4 |
| ��Һ�����/mL | 10.00 | 10.00 | 10.00 | 10.00 |
| ����I2����Һ�����/mL | 19.99 | 19.98 | 17.13 | 20.03 |
�������һ��I2����Һ����Һ�������Ұ��������ɫ���ı�
�������һ��I2����Һ����Һ�������Ұ��������ɫ���ı�
����Na2S2O3?5H2O�ڲ�Ʒ�е����������ǣ�����������1λС����
90.2%
90.2%
����Na2S2O3?5H2O��ʽ��Ϊ248����������1����ͭ��Ũ���ᷴӦ��������ͭ�Ͷ����������壻
�ڸ�����ĿNa2S2O3��������Һ�в����ȶ����ڵ���Ϣ�жϣ�
�۸��ݹ��˲������������жϣ�
�ܶ����������ŷŵ������У�Ӧ��β������װ�ã�
��2���ٴﵽ�ζ��յ����������Һ�������Ұ���Ӳ��ı䣻
�ڸ��ݷ�Ӧ�Ĺ�ϵʽ���㣮
�ڸ�����ĿNa2S2O3��������Һ�в����ȶ����ڵ���Ϣ�жϣ�
�۸��ݹ��˲������������жϣ�
�ܶ����������ŷŵ������У�Ӧ��β������װ�ã�
��2���ٴﵽ�ζ��յ����������Һ�������Ұ���Ӳ��ı䣻
�ڸ��ݷ�Ӧ�Ĺ�ϵʽ���㣮
����⣺��1����Ũ�������ǿ�����ԣ��ڼ��ȵ�����������������ͭ����Ӧ�Ļ�ѧ����ʽ��Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O��
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O��
�����ɵ�SO2��̼���Ʒ�Ӧ�����������ƺ�CO2������Na2S2O3��������Һ�в����ȶ����ڣ�����C��̼���Ƶ���������Ӧ�����ü��ṩ���Ի�������ֹͣC�еķ�Ӧ���IJ����Ǵ�K2���ر�K1��
�ʴ�Ϊ��Na2S2O3��������Һ�в����ȶ����ڣ���K2���ر�K1��
�۹���ʱ����Ҫ����������©�����ձ������������ʴ�Ϊ��©�������������ձ���
��SO2�Ǵ�����Ⱦ���Ҫβ����������װ��B��ʢ�ŵ��Լ�������������Һ����������SO2����ֹ��Ⱦ������
�ʴ�Ϊ��NaOH����C�еķ�Ӧֹͣ������A�в����Ķ���SO2����ֹ������Ⱦ��
��2�������ڵ�����������ɫ����ζ�ʱ���ﵽ�ζ��յ����������Һ�������Ұ���Ӳ��ı䣬
�ʴ�Ϊ���������һ��I2����Һ����Һ�������Ұ��������ɫ���ı䣻
�ڸ��ݱ������ݿ�֪����3��ʵ��������I2����Һ�����ƫС����ȥ����ʵ������I2����Һ�������ƽ��ֵ��
mL=20.0mL������ݷ�Ӧ�ķ���ʽ��֪��Na2S2O3?5H2O�����ʵ�����0.050mol/L��0.0200L��2��10=0.02mol������Na2S2O3?5H2O�ڲ�Ʒ�е�����������
��100%=90.2%��
�ʴ�Ϊ��90.2%��
| ||
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
| ||
�����ɵ�SO2��̼���Ʒ�Ӧ�����������ƺ�CO2������Na2S2O3��������Һ�в����ȶ����ڣ�����C��̼���Ƶ���������Ӧ�����ü��ṩ���Ի�������ֹͣC�еķ�Ӧ���IJ����Ǵ�K2���ر�K1��
�ʴ�Ϊ��Na2S2O3��������Һ�в����ȶ����ڣ���K2���ر�K1��
�۹���ʱ����Ҫ����������©�����ձ������������ʴ�Ϊ��©�������������ձ���
��SO2�Ǵ�����Ⱦ���Ҫβ����������װ��B��ʢ�ŵ��Լ�������������Һ����������SO2����ֹ��Ⱦ������
�ʴ�Ϊ��NaOH����C�еķ�Ӧֹͣ������A�в����Ķ���SO2����ֹ������Ⱦ��
��2�������ڵ�����������ɫ����ζ�ʱ���ﵽ�ζ��յ����������Һ�������Ұ���Ӳ��ı䣬
�ʴ�Ϊ���������һ��I2����Һ����Һ�������Ұ��������ɫ���ı䣻
�ڸ��ݱ������ݿ�֪����3��ʵ��������I2����Һ�����ƫС����ȥ����ʵ������I2����Һ�������ƽ��ֵ��
| 19.99+19.98+20.03 |
| 3 |
| 0.02mol��248g/mol |
| 5.5g |
�ʴ�Ϊ��90.2%��
���������⿼�����ʵĺ����IJⶨ��ͭ��Ũ����ķ�Ӧ��������Ʊ���֪ʶ���Ǹ߿��еij�����������ͣ������е��Ѷ�����Ŀ��飬�����ۺ���ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ���������������淶�Ͻ���ʵ��������������ѧ����ѧ��������������Ҫ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ�����������������������ѧ���淶�Ͻ���ʵ����������Լ�����������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ