��Ŀ����
I����A��B��C��D���ֶ�����Ԫ�أ�����A��Dͬ���壻����֪B��A���γ����ΪBA�Ļ��������A�Ļ��ϼ�Ϊ-1��B��C���γ����ΪB2C2�Ļ����A��B��C�γɵĵ������ӵĺ������������ͬ��
��1��Ԫ��A�����ڱ��е�λ���� ��
��2��B��C��D���γ����ΪBDC�Ļ�����û�����ˮ��Һ��ͨ�����CO2������Ӧ�����ӷ���ʽΪ ��
��3��B2C2�����������¿��γɾ��ж�Ԫ�������ʵ����ʣ������������ʺ�B������������Ӧˮ���ﷴӦʱ������һ����ʽ�Σ�����ʽ�εĵ���ʽΪ ��
II����������һֱ��Ϊ���ĺ�������ڡ�������1971��˹ͼ�ܶ��Ͱ��������������ɹ��غϳ��˴η���������۵㱻���ҵض�ҡ�ˡ���������0�����½��������ϸ��ĩ������ͨ�����õ��������Ĵη��ᡣ��֪�η���ķ��ӹ������������¡�
�Ŵη�������Ԫ�صĻ��ϼ�Ϊ ��
����������˼������Ӻͻ��Ż�ѧ���ļ��ܣ�E����
| H2 | O2 | F2 | O-H | O-F | H-F | |
| E/(kJ/mol) | 432 | 494 | 155 | 424 | 220 | 566 |
��14�֣�
��1���ڶ����ڡ���A ��1�֣�
��2�� ClO��+H2O+CO2==HClO+HCO3����2�֣�
��3��![]() ��2�֣�
��2�֣�
��1��0 (1��)
��2��-338 (2��)
��3��HOF+H2O���ȣ�=H2O2+HF (2��)
��4��+4 +3 ( 2��) 1 (2��)

 ��У����ϵ�д�
��У����ϵ�д���14�֣�
I����A��B��C��D���ֶ�����Ԫ�أ�����A��Dͬ���壻����֪B��A���γ����ΪBA�Ļ��������A�Ļ��ϼ�Ϊ-1��B��C���γ����ΪB2C2�Ļ����A��B��C�γɵĵ������ӵĺ������������ͬ��
��1��Ԫ��A�����ڱ��е�λ���� ��
��2��B��C��D���γ����ΪBDC�Ļ�����û�����ˮ��Һ��ͨ�����CO2������Ӧ�����ӷ���ʽΪ ��
��3��B2C2�����������¿��γɾ��ж�Ԫ�������ʵ����ʣ������������ʺ�B������������Ӧˮ���ﷴӦʱ������һ����ʽ�Σ�����ʽ�εĵ���ʽΪ ��
II����������һֱ��Ϊ���ĺ�������ڡ�������1971��˹ͼ�ܶ��Ͱ��������������ɹ��غϳ��˴η���������۵㱻���ҵض�ҡ�ˡ���������0�����½��������ϸ��ĩ������ͨ�����õ��������Ĵη��ᡣ��֪�η���ķ��ӹ������������¡�
�Ŵη�������Ԫ�صĻ��ϼ�Ϊ ��
����������˼������Ӻͻ��Ż�ѧ���ļ��ܣ�E����
|
| H2 | O2 | F2 | O-H | O-F | H-F |
| E/(kJ/mol) | 432 | 494 | 155 | 424 | 220 | 566 |
����㷴Ӧ��2HFO=2HF+O2�ķ�Ӧ��(��H)�Ľ���ֵΪ kJ/mol��
�Ǵη���ɲ�Ǽ��ܱ���ˮ���ֽ⣬����һ�ֳ���������H2O2��д���η�������ˮ��Ӧ�Ļ�ѧ����ʽ�� ��
��4��1986�꣬��ѧ��KarlChriste�״���2K2MnF6 +4SbF5 === 4KSbF6 + 2MnF3 + F2����ѧ�����Ƶ���F2���÷�Ӧ�б���ԭ��Ԫ�ػ��ϼ۴� �۱�Ϊ �ۣ�����Ӧ�����ɱ�״����11.2 L��F2������ mol���ӷ���ת�ơ�
��14�֣�
I����A��B��C��D���ֶ�����Ԫ�أ�����A��Dͬ���壻����֪B��A���γ����ΪBA�Ļ��������A�Ļ��ϼ�Ϊ-1��B��C���γ����ΪB2C2�Ļ����A��B��C�γɵĵ������ӵĺ������������ͬ��
��1��Ԫ��A�����ڱ��е�λ���� ��
��2��B��C��D���γ����ΪBDC�Ļ�����û�����ˮ��Һ��ͨ�����CO2������Ӧ�����ӷ���ʽΪ ��
��3��B2C2�����������¿��γɾ��ж�Ԫ�������ʵ����ʣ������������ʺ�B������������Ӧˮ���ﷴӦʱ������һ����ʽ�Σ�����ʽ�εĵ���ʽΪ ��
II����������һֱ��Ϊ���ĺ�������ڡ�������1971��˹ͼ�ܶ��Ͱ��������������ɹ��غϳ��˴η���������۵㱻���ҵض�ҡ�ˡ���������0�����½��������ϸ��ĩ������ͨ�����õ��������Ĵη��ᡣ��֪�η���ķ��ӹ������������¡�
�Ŵη�������Ԫ�صĻ��ϼ�Ϊ ��
����������˼������Ӻͻ��Ż�ѧ���ļ��ܣ�E����
|
|
H2 |
O2 |
F2 |
O-H |
O-F |
H-F |
|
E/(kJ/mol) |
432 |
494 |
155 |
424 |
220 |
566 |
����㷴Ӧ��2HFO=2HF+O2�ķ�Ӧ��(��H)�Ľ���ֵΪ kJ/mol��
�Ǵη���ɲ�Ǽ��ܱ���ˮ���ֽ⣬����һ�ֳ���������H2O2��д���η�������ˮ��Ӧ�Ļ�ѧ����ʽ�� ��
��4��1986�꣬��ѧ��Karl Christe�״���2K2MnF6 + 4SbF5 === 4KSbF6 + 2MnF3 + F2����ѧ�����Ƶ���F2���÷�Ӧ�б���ԭ��Ԫ�ػ��ϼ۴� �۱�Ϊ �ۣ�����Ӧ�����ɱ�״����11.2 L��F2������ mol���ӷ���ת�ơ�
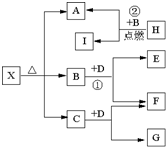 ����A��B��C��D��E���ֶ�����Ԫ�أ����ǵ�ԭ��������������AԪ�ص�ԭ���ǰ뾶��С��ԭ�ӣ�BԪ�ص�����������ˮ���������⻯�ﷴӦ����һ����X��D��Aͬ�壬����Eͬ���ڣ�EԪ�ص������������Ǵ�����������
����A��B��C��D��E���ֶ�����Ԫ�أ����ǵ�ԭ��������������AԪ�ص�ԭ���ǰ뾶��С��ԭ�ӣ�BԪ�ص�����������ˮ���������⻯�ﷴӦ����һ����X��D��Aͬ�壬����Eͬ���ڣ�EԪ�ص������������Ǵ����������� NH3?H2O+H+
NH3?H2O+H+