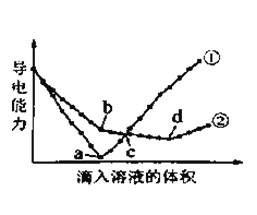
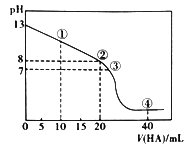
��Ŀ����
����Ŀ���������ֶ�����Ԫ�آ�H ��C ��N ��O ��Na ��Cl��
��1��������γɵĻ�����ĵ���ʽ��__________����ҵ�����øû������Ʊ�NO�Ļ�ѧ����ʽ___________��
��2���ݵĵ����ڢܵĵ�����ȼ�գ����ɵ���ɫ���塣�ò���ĵ���ʽ��______________________________���ò����к��еĻ�ѧ����������______________________________��
��3���ں͢���̬�⻯�������ȶ��Խ�ǿ����__________���û�ѧʽ��ʾ�����ܱ�ʾ���ں͢����������ˮ���������ǿ���Ļ�ѧ����ʽ��______________________________��
���𰸡� ![]() 4NH3+5O2
4NH3+5O2![]() 2N2+6H2O
2N2+6H2O ![]() �Ǽ��Թ��ۼ������Ӽ� HCl Na2CO3+2HClO4=2NaClO4+H2O+CO2��
�Ǽ��Թ��ۼ������Ӽ� HCl Na2CO3+2HClO4=2NaClO4+H2O+CO2��
�������� (1)H��N�γɵĻ�����NH3Ϊ���ۻ���������ʽ��![]() ������NH3�Ļ�ԭ�ԣ�ͨ�����Ĵ������ɵõ�NO��������Ӧ�Ļ�ѧ����ʽΪ4NH3+5O2
������NH3�Ļ�ԭ�ԣ�ͨ�����Ĵ������ɵõ�NO��������Ӧ�Ļ�ѧ����ʽΪ4NH3+5O2![]() 2N2+6H2O��
2N2+6H2O��
(2)Na��������ȼ�գ����ɵ���ɫ����Na2O2�������ӻ�����ĵ���ʽ��![]() �����еĻ�ѧ���������ǷǼ��Թ��ۼ������Ӽ���
�����еĻ�ѧ���������ǷǼ��Թ��ۼ������Ӽ���
(3)Cl�ķǽ����Ա�Cǿ����HCl���ȶ��Ա�CH4ǿ������ǿ���������ԭ��������HClO4�����Ա�̼��ǿ�Ļ�ѧ����ʽ�� Na2CO3+2HClO4=2NaClO4+H2O+CO2����

����Ŀ��ijͬѧ�����������������ԭNO3-�ѳ�����ˮ�������Ρ���������Ϻ���������װ��̽��������KNO3��Һ�ķ�Ӧ��ʵ�鲽�輰�������£�
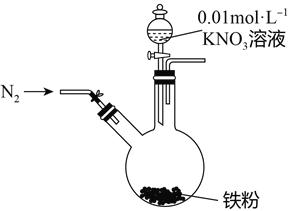
ʵ�鲽�� | ʵ������ |
1�����ɼУ�����ͨ��N2 | |
2������pHΪ2.5��0.01mol/L����KNO3��Һ100mL | ���۲����ܽ⣬��Һ��dz��ɫ�� ���۲����ܽ��ʣ�����۱������������ɫ���ʸ��š� |
3����Ӧֹͣ�ε���������Բ����ƿȡ�� | ��ƿ���������ɫû�з����仯�� |
4����ʣ�������� | ����İ�ɫ���ʱ�Ϊ���ɫ�� |
��1��ͨ��N2�����ֺ�����Ӧ����N2��Χ�н��е�ʵ��Ŀ����______________________________��
��2����ɫ������__________���û�ѧ����ʽ�������Ϊ���ɫ��ԭ��____________________��
��3��Ϊ��̽���Һ�ijɷ֣���ͬѧ��һ�����������ʵ�飺
ʵ�鲽�� | ʵ������ |
1��ȡ������Һ���Թ��У������м���KSCN��Һ | ��ҺҺ�ޱ仯 |
2����������Һ��Ϊ���ݣ�һ���е�����������һ���еμ�ϡ���� | ������Һ����Ϊ��ɫ |
3����ȡ������Һ���Թ��У������м���ŨNaOH��Һ�����ȣ����Թܿڷ���ʪ��ĺ�ɫʯ����ֽ�� | ���������ɣ�������ʹ��ɫʯ����ֽ������ |
��i����������ʵ���������ж���Һ�д���____________________���ӡ�
��ii������2�еμ�ϡ�������Һ����dz��ɫ��ɺ�ɫ���������ӷ���ʽ������ԭ��____________________��