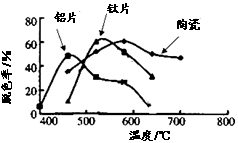
��Ŀ����
�����ƻ�2015���ٴε�½���̶��������Ͻ������أ��������½����������Ϊ������ʵ�����룬1992�����������˵�һ������ʵ�ʵ�������ʯ��������������(FeTiO3����������)����������������������顣�о����֣������ϣ������о������˼ס�����λѧ���ļ�����Ȥ����������֪�������ܱ�C��ԭ��
2FeTiO3+C![]() 2Fe+2TiO2+CO2��
2Fe+2TiO2+CO2��
���Ƿֱ����������ʵ��װ��ģ��ⶨ�������п���ȡ��������������ʵ�顣
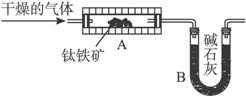
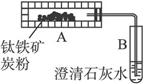
ͼ1 ͼ2
(1)ѧ����������ͼ1װ��(AΪ�����װ��)������������ȡ������д�������ʵ�鲽�裺____________(ѡ���������ֱ��)��
��ͨ��N2 ��ֹͣͨN2 ��ͨ��H2 ��ֹͣͨH2 �ݼ��� ��ֹͣ���� �߳�ȡװ��B��������
(2)ѧ����������ͼ2װ��(AΪ�����װ��)������������ȡ����������Ϊ�÷������������ڣ���Ӧ������CO2��ͨ����ɫֲ��Ĺ������ת��ΪӪ�����ʣ�ͬʱ��������(6O2+6H2O![]() C6H12O6+6O2)��ʵ������У��Ƶ÷�Ӧǰװ��A�������������Ϊa g��̿�۵�����Ϊb g����Ӧ��װ��B�в���CaCO3������Ϊc g�����������п���ȡ�������������ı���ʽΪ____________��
C6H12O6+6O2)��ʵ������У��Ƶ÷�Ӧǰװ��A�������������Ϊa g��̿�۵�����Ϊb g����Ӧ��װ��B�в���CaCO3������Ϊc g�����������п���ȡ�������������ı���ʽΪ____________��
(3)��ר��������϶������������������壬��ʵ����Ʒ��滹���ڲ���֮��������ͼ2װ���ڼ���ʱ���������CO����ʵ��������������ͼ2��ʾװ��Ӧ��ȡ�ĸĽ���ʩ��__________���Ľ���ʵ����ʼ�����������2װ��A��ͨ��N2��Ŀ����_____________��
(4)��ѧ����ѡ��H2������CO����ԭ����ԭ���ǣ�______________________________��
(1)�ߢۢݢޢܢ�
(2)![]() ��100%
��100%
(3)����ͼ1װ�ã�����B֮������ʢ��CuO�۵�ȼ�չ�(��ȡ������)�������
���Ƚ�װ���еĿ����ϳ�����Ӧ��ʼ��ɽ�CO2��ʱ�ϳ�ʹ֮����ʯ������
(4)H2����������ˮ����������������������ʹH2�ܹ���ѭ������
������(1)����ͨH2��װ���ڵĿ����ų�������H2��O2��Ӧ����ˮ�����ɵ�ˮ����ʯ�����ղ��������������H2O���������Ӷ����O2����������˳��Ϊ�ߢۢݢޢܢߡ�
(2)����������Ϊ![]() ��
��![]() g
g
���������п���ȡ������������Ϊ![]() 100%��
100%��
(4)��ѧ��ѡ��H2������CO����ΪH2����������ˮ�����������O2��H2��ʹ��H2�ܹ���ѭ�����á�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д� 2NH3(g)����Ӧ�ﵽƽ����NH3�����ʵ���Ϊamol
2NH3(g)����Ӧ�ﵽƽ����NH3�����ʵ���Ϊamol


 2NH3(g)����Ӧ�ﵽƽ����NH3�����ʵ���Ϊamol
2NH3(g)����Ӧ�ﵽƽ����NH3�����ʵ���Ϊamol



 C6H12O6�������ǣ�+6O2��ʵ������У��Ƶ÷�Ӧǰװ��A�������������Ϊag��̿�۵�����Ϊbg����Ӧ��װ��B�в���CaCO3������Ϊcg�����������п���ȡ�������������ı���ʽΪ________________��
C6H12O6�������ǣ�+6O2��ʵ������У��Ƶ÷�Ӧǰװ��A�������������Ϊag��̿�۵�����Ϊbg����Ӧ��װ��B�в���CaCO3������Ϊcg�����������п���ȡ�������������ı���ʽΪ________________��