��Ŀ����
��7�֣���֪A��B��C��D��E�Ƕ������е����ַǽ���Ԫ�أ����ǵ�ԭ��������������AԪ��ԭ���γɵ����Ӿ���һ�����ӣ�C��D��Ԫ�����ڱ��д������ڵ�λ�ã�Bԭ�ӵ��������������ڲ��������2����EԪ����DԪ��ͬ���壻E�ĵ���Ϊ��ɫ���壬������CS2
��1����д��Ԫ�ط��ţ�B ��C ��
��2������E�������ӽṹʾ��ͼ�� ��
��3��A���ʺ�C������һ�������·�Ӧ���ɻ�����X���÷�Ӧ�Ļ�ѧ����ʽΪ
��
��4����9gB������������D������ȼ�գ���������ͨ��1L 1mol/L��NaOH��Һ�У���
ȫ���պ���Һ��(��Na+��)Ũ������������ ��
(1) C N ��
������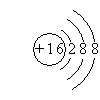
��3����2������2  ���Σ�3��2�֣�
���Σ�3��2�֣�
��4��HCO3��(����ʽ2�֣�����ÿ��1��)
����

��ϰ��ϵ�д�
�����Ŀ
��֪A��B��C��D�ֱ���Cu��Ag��Fe��Al���ֽ����е�һ�֣���֪��A��C������ϡ���ᷴӦ�ų����壻��B��D�������η�Ӧ���û�������D����C��ǿ�Ӧ�ų����壬�ɴ˿����ƶ�A��B��C��D�����ǣ�������
| A��Fe��Cu��Al��Ag | B��Al��Cu��Fe��Ag | C��Cu��Ag��Al��Fe | D��Ag��Al��Cu��Fe |

 ��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ� ����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�