��Ŀ����
����Ŀ��������������������⣺
��1����ʵ��ʱƤ���ϲ�С��ճ��һЩ������أ��γɵĺڰߺܾò�������������ò����ϡ��Һϴ�����Ͽ��Ը�ԭ�������ӷ���ʽΪ��MnO4- + C2O42- + H+ �� CO2��+ Mn2+ + ![]()
�ٸ÷�Ӧ����������_____�ұ߷����ڵIJ�����_____
����ɲ���ƽ�����ӷ���ʽ __________________����ת��������_____ e-
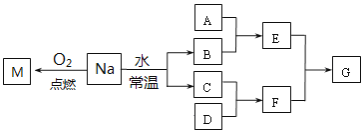
��2����ͼ��ʾij��̬����A���仯����֮���ת����ϵ��ijЩ����ͷ�Ӧ��������ȥ��������A����ij��̬���ʻ������ɻ�����B��
��������B������������Ҫ��Ⱦ�������D����ɵ���AԪ�ص�����������Ӧˮ�������A��_________���������ƣ�����������Bͨ����ˮ�й۲쵽��������_________ ��������˵��������B����_______ �ԡ�
���𰸡�MnO4-H2O2 MnO4- +5 C2O42- + 16 H+ == 10 CO2��+ 2 Mn2+ + 8H2O10�����ˮ��ɫ��ԭ��
��������
��1��MnO4- + C2O42- + H+ �� CO2��+ Mn2+ + ![]() ��MnԪ�صĻ��ϼ���+7�۽���Ϊ+2����CԪ�صĻ��ϼ���+3������Ϊ+4�����ɵ����غ����֪����2MnO4- + 5C2O42- + H+ ��10 CO2��+ 2Mn2+ +
��MnԪ�صĻ��ϼ���+7�۽���Ϊ+2����CԪ�صĻ��ϼ���+3������Ϊ+4�����ɵ����غ����֪����2MnO4- + 5C2O42- + H+ ��10 CO2��+ 2Mn2+ + ![]() �����ɵ���غ����֪����2MnO4- + 5C2O42- + 6H+ ��10 CO2��+ 2Mn2+ +
�����ɵ���غ����֪����2MnO4- + 5C2O42- + 6H+ ��10 CO2��+ 2Mn2+ + ![]() ����ԭ���غ����֪�����÷�ӦΪ2MnO4- + 5C2O42- + 6H+ ��10 CO2��+ 2Mn2+ +8H2O ���Դ��������
����ԭ���غ����֪�����÷�ӦΪ2MnO4- + 5C2O42- + 6H+ ��10 CO2��+ 2Mn2+ +8H2O ���Դ��������
��2������������Ҫ��Ⱦ����SO2������B��SO2����A�ǵ���S��C��SO3��D��H2SO4��E������H2SO3������������SO2���л�ԭ�����ܱ���ˮ��������ʹ��ˮ��ɫ��
��1��MnO4- + C2O42- + H+ �� CO2��+ Mn2+ + ![]() ��MnԪ�صĻ��ϼ���+7�۽���Ϊ+2����CԪ�صĻ��ϼ���+3������Ϊ+4�����ɵ����غ����֪����2MnO4- + 5C2O42- + H+ ��10 CO2��+ 2Mn2+ +
��MnԪ�صĻ��ϼ���+7�۽���Ϊ+2����CԪ�صĻ��ϼ���+3������Ϊ+4�����ɵ����غ����֪����2MnO4- + 5C2O42- + H+ ��10 CO2��+ 2Mn2+ + ![]() �����ɵ���غ����֪����2MnO4- + 5C2O42- + 6H+ ��10 CO2��+ 2Mn2+ +
�����ɵ���غ����֪����2MnO4- + 5C2O42- + 6H+ ��10 CO2��+ 2Mn2+ + ![]() ����ԭ���غ����֪�����÷�ӦΪ2MnO4- + 5C2O42- + 6H+ ��10 CO2��+ 2Mn2+ +8H2O �������÷�Ӧ��֪��
����ԭ���غ����֪�����÷�ӦΪ2MnO4- + 5C2O42- + 6H+ ��10 CO2��+ 2Mn2+ +8H2O �������÷�Ӧ��֪��
�ٸ÷�Ӧ����������MnO4- ���ұ߷����ڵIJ�����H2O��
�ڸ����ӷ���ʽ2 MnO4- +5C2O42- +16 H+ =10CO2��+2Mn2+ +8H2O������ת��������10e-��
��ˣ�������ȷ��Ϊ��MnO4- ��H2O ��2 MnO4- +5 C2O42- + 16 H+ =10 CO2��+ 2 Mn2+ + 8 H2O��10 ��
��2������������Ҫ��Ⱦ����SO2������B��SO2����A�ǵ���S��C��SO3��D��H2SO4��E������H2SO3������������SO2���л�ԭ�����ܱ���ˮ��������ʹ��ˮ��ɫ��
��ˣ�������ȷ�������������ˮ��ɫ����ԭ�ԡ�

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�����Ŀ���±�����ʾ���ʻ�����Ĵ�����ϵ������Ȧ����

X | Y | Z | |
A | ��Ԫ�� | ����Ԫ�� | ������Ԫ�� |
B | ����� | ������ | ������ |
C | �������� | ��ɢϵ | ���� |
D | �û���Ӧ | ������ԭ��Ӧ | ���ȷ�Ӧ |
A. A B. B C. C D. D