��Ŀ����
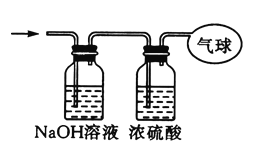
����Ŀ��ijѧϰС������ͼ��ʾװ�òⶨпͭ�Ͻ���п��ͭ������������

��1��ʵ��ǰ���Ƚ�пͭ�Ͻ���ϡ�����н���Ƭ�̣���Ŀ���ǣ�___________________________________��
��2��ʵ����������У�����ҩƷ��ˮװ���������
�����Ӻ�װ�ú��������
����¼C��Һ��λ��
����B��ʣ�������ˣ�ϴ�ӣ��������
����B�в���������������ָ������º�
��¼C��Һ��λ��
����A��B�μ�������ϡ����
����ʵ�����ȷ����˳����________________������ţ�����¼C��Һ��λ��ʱ��������ƽ���⣬��Ӧ_____________________
��3��B�з�����Ӧ�Ļ�ѧ����ʽΪ_________________ ��
��4����ʵ����пͭ�Ͻ������Ϊa g����ϡ�����ַ�Ӧ��
B��ʣ����������Ϊbg����п����������Ϊ____________��
��5��ʵ������У���δϴ�ӹ������õIJ��������п������������______������ƫ������ƫС����������Ӱ��������
���𰸡���ȥ�Ͻ���������Ĥ �ڢ٢ۢޢݢ� ʹD��C��Һ����ƽ Zn + H2SO4 = ZnSO4+H2�� (a��b)/a��100% ƫС
��������
(1)ʵ��ǰ,�Ƚ�пͭ�Ͻ���ϡ���н���Ƭ��,��ȥ�Ͻ���������Ĥ;
(2)
��ʵ����Ҫ���������������������ʵ�����Ӻ�װ�ú�������Ԣڣ�Ȼ��ҩƷ��ˮװ��������Т�����A��B�μ�������ϡ����Ҫ�����ſ�Һ���������ⶨ�������������,�������¼C��Һ��,Ȼ��ʹ��Ӧ����,����ַ�Ӧʱ�ڼ���C��λ�â�,Ȼ����A��B�μ�������ϡ���Ὺʼ��Ӧ�ޣ���Ӧ�����ⶨ��������ݣ����ⶨδ��Ӧ���������ܣ����Ա����Ϊ:�ڢ٢ۢޢݢ�;��¼C��Һ��λ��ʱ,������ƽ����,��Ӧ ʹD��C��Һ����ƽ��
(3)пͭ�Ͻ���п��ϡ���ᷴӦ����������ͭ����ϡ���ᷴӦ���ʷ�Ӧ����ʽΪ��Zn + H2SO4 = ZnSO4+H2����
(4)ͭп�Ͻ������Ϊag,B��ʣ����������Ϊbg,��п������Ϊ(a-b)g,�Ӷ��������п����������Ϊ(a-b)/a��100%��
(5)ʵ������У���δϴ�ӹ������õIJ������ⶨʣ�����ͭ������ƫ�����Բ��п������������ƫС��

����Ŀ��(1)�Ķ������������������ϣ�
����һ

���϶�
���� | �۵�/�� | �е�/�� | �ܶ�/ g/cm3 | �ܽ��� |
�Ҷ���(C2H6O2) | ��11.5 | 198 | 1.11 | ������ˮ���Ҵ� |
������(C3H8O3) | 17.9 | 290 | 1.26 | �ܸ�ˮ���ƾ�������Ȼ��� |
�ش���������(����ĸ���)��
A�������������������������� B����ȡ��
C�����ܽ⡢�ᾧ���������ķ��� D����Һ��
�ٽ�������Ȼ��ƺʹ���Ļ�����з�����������Ӧ��________��
�ڽ��Ҷ����ͱ�������������ѷ�����________________________��
(2)�Ķ�������
���ܽ��Է��棬Br2(��)��I2�����ƣ���ϡ��ˮ��Һ�Ի�ɫ����ʵ���������ˮ(Br2��ˮ��Һ)����ȡBr2����ȡI2�ķ������ơ�
�ش��������⣺
�ٳ��õ���ȡ������________________����ѧ�Լ���________������Ҫ��������__________��
�����۲췢����ȡBr2�Ժ��ˮ������ɫ�����������ķ�����__________________��