��Ŀ����
����ˮ���ʵ�鲽�����£�
(1)����֧�Թ��зֱ����4 mL������Һ��
(2)������һ֧�Թ��м����ˮ���۲쵽��������_________________________
______________________________________��
(3)��һ֧�Թ��м�������ϡ���ᣬ����4��5 min��Ȼ����NaOH��Һ�к�ϡ���ᡣ�ѵõ�����Һ��װ����֧�Թ�a��b�У�a�Թ��м����ˮδ������������b�Թ��м������Ƶĺ�NaOH��Һ��Cu(OH)2����Һ�������������ڳ���ש��ɫ������������ʵ���������ܵó��Ľ�����____________________________________________________________________________________________________��
(4)��ʵ�鲽��(3)�У�ϡ�����ڵ���ˮ�����������ʲô���ã�_____________________________________________________________________________��
(5)Ϊʲô�ڼ������ˮ�����ɵ�������֮ǰҪ����NaOH��Һ�к�ˮ���Ļ��Һ��________________________________________________________________________________________________________________________________��
(6)д������ˮ��Ļ�ѧ����ʽ��__________________________________________________________________________________________________________��
���𰸡�(2)����ɫ (3)��������ȫˮ��
(4)����
(5)����ˮ����ϡ����������,������������Ӧ�ڼ��������½���
(6)(C6H10O5)n(����)+nH2O��nC6H12O6(������)
�����������������������ɫ���ݴ˶���֮�������飻������ϡ����Ĵ��¿�ˮ��Ϊ�����ǣ������ǿ�ʹ���Ƶ�������ͭ����Һ��ԭΪש��ɫ������ͭ�����Ǽ��������ǵ���Ч��Ӧ���������Ƶ�������ͭ����Һ����������������µ�ˮ�����ʱ�����ü��к����е��ᡣ

 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�PCl3�����ڰ뵼�����������ӡ���ɢ�����й����ʵIJ����������£�
|
|
�۵�/�� |
�е�/�� |
�ܶ�/g��mL��1 |
���� |
|
���� |
44��1 |
280��5 |
1��82 |
2P(����)+3Cl2 |
|
PCl3 |
��112 |
75��5 |
1��574 |
��ˮ����H3PO3��HCl����O2����POCl3 |
|
POCl3 |
2 |
105��3 |
1��675 |
��ˮ����H3PO4��HCl��������PCl3 |
��ͼ��ʵ�����Ʊ�PCl3��װ�ã�����������ʡ�ԣ���
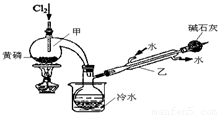
��1�������ͨ������Cl2֮ǰ������ͨ��һ��ʱ��CO2��Ŀ���� ��
��2��ʵ������У�Ӧ���� ���Լ���PCl5�����ɡ�
��3���ֲ�ƷƷ�г�����POCl3��PCl5�ȡ���������ȳ�ȥPCl5��ͨ�� ����ʵ��������ƣ������ɵõ�PCl3�Ĵ�Ʒ��
��4����֪��a��H3PO3+H2O+I2
 H3PO4+2HI��b��(NH4)3BO3��Һ����HI��H3PO4��Ӧ����H3BO3�����ᣩ��c��Na2S2O3��Һ�ɶ����ⶨ�⣺I2+2Na2S2O3��Na2S4O6+2NaI
H3PO4+2HI��b��(NH4)3BO3��Һ����HI��H3PO4��Ӧ����H3BO3�����ᣩ��c��Na2S2O3��Һ�ɶ����ⶨ�⣺I2+2Na2S2O3��Na2S4O6+2NaI
�ٲⶨ��Ʒ��PCl3����������ʵ�����£��벹����Ӧ��ʵ�鲽�裺
����1��Ѹ����ȡm g��Ʒ��ˮ����ȫ����500mL����ƿ�ж��ݡ�
����2��������ƿ����ȡ25��00mL��Һ������ƿ�С�
����3��ȷ����c1 mol/L����ҺV1 mL������������ ��
����4���ñ�Na2S2O3��Һ�صι����ĵ⣬�����յ�ʱ����3 mL������Һ�����������յ㣨��ɫ��Һ��ɫ���������յ�ʱ����c2 mol/L Na2S2O3��ҺV2 mL��
�ڸ����������ݣ��ò�Ʒ��PCl3����������Ϊ ���ú���ĸ�Ĵ���ʽ��ʾ����
PCl3�����ڰ뵼�����������ӡ���ɢ�����й����ʵIJ����������£�
|
|
�۵�/�� |
�е�/�� |
�ܶ�/g��mL��1 |
���� |
|
���� |
44��1 |
280��5 |
1��82 |
2P(����)+3Cl2 |
|
PCl3 |
��112 |
75��5 |
1��574 |
��ˮ����H3PO3��HCl����O2����POCl3 |
|
POCl3 |
2 |
105��3 |
1��675 |
��ˮ����H3PO4��HCl��������PCl3 |
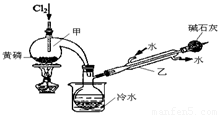
��ͼ��ʵ�����Ʊ�PCl3��װ�ã�����������ʡ�ԣ���

��1�������ͨ������Cl2֮ǰ������ͨ��һ��ʱ��CO2��Ŀ���� ��
��2��ʵ������У�Ӧ���� ���Լ���PCl5�����ɡ�
��3���ֲ�ƷƷ�г�����POCl3��PCl5�ȡ���������ȳ�ȥPCl5��ͨ�� ����ʵ��������ƣ������ɵõ�PCl3�Ĵ�Ʒ��
��4����֪��a��H3PO3+H2O+I2
 H3PO4+2HI��b��(NH4)3BO3��Һ����HI��H3PO4��Ӧ����H3BO3�����ᣩ��c��Na2S2O3��Һ�ɶ����ⶨ�⣺I2+2Na2S2O3��Na2S4O6+2NaI
H3PO4+2HI��b��(NH4)3BO3��Һ����HI��H3PO4��Ӧ����H3BO3�����ᣩ��c��Na2S2O3��Һ�ɶ����ⶨ�⣺I2+2Na2S2O3��Na2S4O6+2NaI
�ٲⶨ��Ʒ��PCl3����������ʵ�����£��벹����Ӧ��ʵ�鲽�裺
����1��Ѹ����ȡm g��Ʒ��ˮ����ȫ����500mL����ƿ�ж��ݡ�
����2��������ƿ����ȡ25��00mL��Һ������ƿ�С�
����3��ȷ����c1 mol/L����ҺV1 mL������������ ��
����4���ñ�Na2S2O3��Һ�صι����ĵ⣬�����յ�ʱ����3 mL������Һ�����������յ㣨��ɫ��Һ��ɫ���������յ�ʱ����c2 mol/L Na2S2O3��ҺV2 mL��
�ڸ����������ݣ��ò�Ʒ��PCl3����������Ϊ ���ú���ĸ�Ĵ���ʽ��ʾ����
PCl3�����ڰ뵼�����������ӡ���ɢ�����й����ʵIJ����������£�
|
|
�۵�/�� |
�е�/�� |
�ܶ�/g��mL��1 |
���� |
|
���� |
44��1 |
280��5 |
1��82 |
2P(����)+3Cl2 |
|
PCl3 |
��112 |
75��5 |
1��574 |
��ˮ����H3PO3��HCl����O2����POCl3 |
|
POCl3 |
2 |
105��3 |
1��675 |
��ˮ����H3PO4��HCl��������PCl3 |
��ͼ��ʵ�����Ʊ�PCl3��װ�ã�����������ʡ�ԣ���
��1�������ͨ������Cl2֮ǰ������ͨ��һ��ʱ��CO2��Ŀ���� ��
��2��ʵ������У�Ӧ���� ���Լ���PCl5�����ɡ�
��3���ֲ�ƷƷ�г�����POCl3��PCl5�ȡ���������ȳ�ȥPCl5��ͨ�� ����ʵ��������ƣ������ɵõ�PCl3�Ĵ�Ʒ��
��4����֪��a��H3PO3+H2O+I2
 H3PO4+2HI��b��(NH4)3BO3��Һ����HI��H3PO4��Ӧ����H3BO3�����ᣩ��c��Na2S2O3��Һ�ɶ����ⶨ�⣺I2+2Na2S2O3��Na2S4O6+2NaI
H3PO4+2HI��b��(NH4)3BO3��Һ����HI��H3PO4��Ӧ����H3BO3�����ᣩ��c��Na2S2O3��Һ�ɶ����ⶨ�⣺I2+2Na2S2O3��Na2S4O6+2NaI
�ٲⶨ��Ʒ��PCl3����������ʵ�����£��벹����Ӧ��ʵ�鲽�裺
����1��Ѹ����ȡm g��Ʒ��ˮ����ȫ����500mL����ƿ�ж��ݡ�
����2��������ƿ����ȡ25��00mL��Һ������ƿ�С�
����3��ȷ����c1 mol/L����ҺV1 mL������������ ��
����4���ñ�Na2S2O3��Һ�صι����ĵ⣬�����յ�ʱ����3 mL������Һ�����������յ㣨��ɫ��Һ��ɫ���������յ�ʱ����c2 mol/L Na2S2O3��ҺV2 mL��
�ڸ����������ݣ��ò�Ʒ��PCl3����������Ϊ ���ú���ĸ�Ĵ���ʽ��ʾ����
PCl3�����ڰ뵼�����������ӡ���ɢ�����й����ʵIJ����������£�
|
|
�۵�/�� |
�е�/�� |
�ܶ�/g��mL��1 |
���� |
|
���� |
44��1 |
280��5 |
1��82 |
2P(����)+3Cl2 |
|
PCl3 |
��112 |
75��5 |
1��574 |
��ˮ����H3PO3��HCl����O2����POCl3 |
|
POCl3 |
2 |
105��3 |
1��675 |
��ˮ����H3PO4��HCl��������PCl3 |
��ͼ��ʵ�����Ʊ�PCl3��װ�ã�����������ʡ�ԣ���
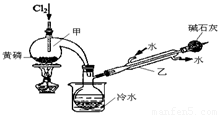
��1�������ͨ������Cl2֮ǰ������ͨ��һ��ʱ��CO2��Ŀ���� ��
��2��ʵ������У�Ӧ���� ���Լ���PCl5�����ɡ�
��3���ֲ�ƷƷ�г�����POCl3��PCl5�ȡ���������ȳ�ȥPCl5��ͨ�� ����ʵ��������ƣ������ɵõ�PCl3�Ĵ�Ʒ��
��4����֪��a��H3PO3+H2O+I2
 H3PO4+2HI��b��(NH4)3BO3��Һ����HI��H3PO4��Ӧ����H3BO3�����ᣩ��c��Na2S2O3��Һ�ɶ����ⶨ�⣺I2+2Na2S2O3��Na2S4O6+2NaI
H3PO4+2HI��b��(NH4)3BO3��Һ����HI��H3PO4��Ӧ����H3BO3�����ᣩ��c��Na2S2O3��Һ�ɶ����ⶨ�⣺I2+2Na2S2O3��Na2S4O6+2NaI
�ٲⶨ��Ʒ��PCl3����������ʵ�����£��벹����Ӧ��ʵ�鲽�裺
����1��Ѹ����ȡm g��Ʒ��ˮ����ȫ����500mL����ƿ�ж��ݡ�
����2��������ƿ����ȡ25��00mL��Һ������ƿ�С�
����3��ȷ����c1 mol/L����ҺV1 mL������������ ��
����4���ñ�Na2S2O3��Һ�صι����ĵ⣬�����յ�ʱ����3 mL������Һ�����������յ㣨��ɫ��Һ��ɫ���������յ�ʱ����c2 mol/L Na2S2O3��ҺV2 mL��
�ڸ����������ݣ��ò�Ʒ��PCl3����������Ϊ ���ú���ĸ�Ĵ���ʽ��ʾ����
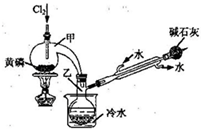 ��2011?�Ͼ���ģ��PCl3�����ڰ뵼�����������ӡ���ɢ�����й����ʵIJ����������£�
��2011?�Ͼ���ģ��PCl3�����ڰ뵼�����������ӡ���ɢ�����й����ʵIJ����������£� 2PCl3��2P+5Cl2(����)
2PCl3��2P+5Cl2(����) 2PCl3��2P+5Cl2(����)
2PCl3��2P+5Cl2(����) 2PCl3��2P+5Cl2(����)
2PCl3��2P+5Cl2(����) 2PCl3��2P+5Cl2(����)
2PCl3��2P+5Cl2(����)