��Ŀ����
����ѧ�뼼����ģ��������һ����ı��أ����Ѻ�ˮ�����ͻ�����������������ȿɽ����ˮ��Դȱ�������⣬�ֿɳ�����ú�����Դ�����Ժ�ˮΪ��Ҫԭ�ϵĺ���ѧ��ҵ���ֱ���Ϊ����ɫ��������
��1�����õĺ�ˮ����������______����______���������������������䶳�������ӽ������ȣ�
��2����ͼ�ǵ�������������ˮ��ԭ��ͼ�����У��缫A��ֱ����Դ���������缫B��ֱ����Դ�ĸ�����
�ٸ�ĤA��______��������ӽ���Ĥ�������ӽ���Ĥ��
�ڴ������۲ɼ��ĺ�ˮ��Ʒ�����������д�����Na+��Cl-���Լ�������K+��SO42-��
��������װ�öԲ��������۵ĺ�ˮ���е�����������������ɺ�A��B��C������������Һ����Һ�壩��pH�ֱ�ΪpHa��pHb��
pHc�������С˳��Ϊ______��
����д���õ��������Բ��������۵ĺ�ˮ���е�������ʱ�������Ļ�ѧ��Ӧ����ʽ______ 2NaOH+H2��+Cl2��

���𰸡���������1����ˮ���������ú�ˮ����������ˮ�Ĺ��̣������ķ����У�����Ĥ�����䶳�������ӽ������ȣ�
��2�������ӽ���Ĥֻ��������������ͨ���������ӽ���Ĥֻ��������������ͨ�������ص������������ӷŵ磬���������������ӷŵ磻���ݵ缫��Ӧ����д�ܵĵ�ⷴӦ��
��3��ˮ������Ҫ�ɷ���������þ��̼��ƣ�
��4�������ӽ�����֬����ʵ��������֮��Ľ��������Ի����£�þ�������γɳ����
����⣺��1����ˮ���������ķ����У�����Ĥ�����䶳�������ӽ������ȣ��ʴ�Ϊ������Ĥ��
��2���������ӽ���Ĥֻ��������������ͨ���������ӽ���Ĥֻ��������������ͨ������ĤA�����������������������ӷŵ磬���Ը�ĤA�������ӽ���Ĥ���ʴ�Ϊ�������ӽ���Ĥ��
�ڵ��ص������������ӷŵ磬���������������ӷŵ磬��ĤA�������ӽ���Ĥ����ĤC�������ӽ���Ĥ������A�������ԣ�B�������ԣ�C���Լ��ԣ�����pH��С˳��Ϊ��pHa��pHb��pHc���ʴ�Ϊ��pHa��pHb��pHc��
�۵���Ȼ��Ƶķ�Ӧԭ��Ϊ��2NaCl+2H2O 2NaOH+H2��+Cl2�����ʴ�Ϊ��2NaCl+2H2O
2NaOH+H2��+Cl2�����ʴ�Ϊ��2NaCl+2H2O 2NaOH+H2��+Cl2����
2NaOH+H2��+Cl2����
��3��ˮ������Ҫ�ɷ���������þ��̼��ƣ�Ҳ�Ǿ�����ʱӲ�ȵ�Ӳˮ�ڳ�ʱ�������к����ɳ�������Ҫ�ɷ֣��ʴ�Ϊ��CaCO3��Mg��OH��2��
��4�������ӽ�����֬����ʵ��������֮��Ľ���������Cl-�������ӽ�����֬��Ӧ�ķ���ʽΪ��RN��CH3��3OH+Cl-�TRN��CH3��3Cl+OH-�����Ի����£�þ�������γɳ��������������ӽ��������������ӽ�����֬��ʱ�������ã��ʴ�Ϊ�������ӽ�����֬��������OH-��Mg2+��Ca2+�ȷ�Ӧ���ɳ������������ӽ�������
������������һ����ѧ�ͼ�������Ŀ���ѶȽϴ���ѧ�������ͽ�������������
��2�������ӽ���Ĥֻ��������������ͨ���������ӽ���Ĥֻ��������������ͨ�������ص������������ӷŵ磬���������������ӷŵ磻���ݵ缫��Ӧ����д�ܵĵ�ⷴӦ��
��3��ˮ������Ҫ�ɷ���������þ��̼��ƣ�
��4�������ӽ�����֬����ʵ��������֮��Ľ��������Ի����£�þ�������γɳ����
����⣺��1����ˮ���������ķ����У�����Ĥ�����䶳�������ӽ������ȣ��ʴ�Ϊ������Ĥ��
��2���������ӽ���Ĥֻ��������������ͨ���������ӽ���Ĥֻ��������������ͨ������ĤA�����������������������ӷŵ磬���Ը�ĤA�������ӽ���Ĥ���ʴ�Ϊ�������ӽ���Ĥ��
�ڵ��ص������������ӷŵ磬���������������ӷŵ磬��ĤA�������ӽ���Ĥ����ĤC�������ӽ���Ĥ������A�������ԣ�B�������ԣ�C���Լ��ԣ�����pH��С˳��Ϊ��pHa��pHb��pHc���ʴ�Ϊ��pHa��pHb��pHc��
�۵���Ȼ��Ƶķ�Ӧԭ��Ϊ��2NaCl+2H2O
 2NaOH+H2��+Cl2�����ʴ�Ϊ��2NaCl+2H2O
2NaOH+H2��+Cl2�����ʴ�Ϊ��2NaCl+2H2O 2NaOH+H2��+Cl2����
2NaOH+H2��+Cl2������3��ˮ������Ҫ�ɷ���������þ��̼��ƣ�Ҳ�Ǿ�����ʱӲ�ȵ�Ӳˮ�ڳ�ʱ�������к����ɳ�������Ҫ�ɷ֣��ʴ�Ϊ��CaCO3��Mg��OH��2��
��4�������ӽ�����֬����ʵ��������֮��Ľ���������Cl-�������ӽ�����֬��Ӧ�ķ���ʽΪ��RN��CH3��3OH+Cl-�TRN��CH3��3Cl+OH-�����Ի����£�þ�������γɳ��������������ӽ��������������ӽ�����֬��ʱ�������ã��ʴ�Ϊ�������ӽ�����֬��������OH-��Mg2+��Ca2+�ȷ�Ӧ���ɳ������������ӽ�������
������������һ����ѧ�ͼ�������Ŀ���ѶȽϴ���ѧ�������ͽ�������������

��ϰ��ϵ�д�
 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д� ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�
�����Ŀ
����ѧ--��ѧ�뼼����
������һ����ı��أ�������Դ�Ŀ��������þ��й�����ǰ����ij�غ�ˮ��pH��7.5��8.6֮�䣬������Ҫ���ӵĺ������±���
��1�����������ǽ��귢չ������һ�ֽϺõĺ�ˮ������������ԭ����ͼ1�������� ���������ӽ���Ĥֻ����������������ͨ����
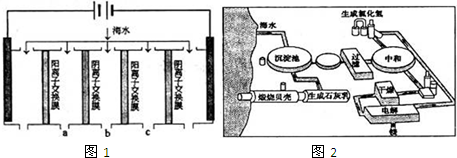
�������ĵ缫��ӦʽΪ ��
�ڵ��һ��ʱ�䣬�����������ˮ������ɷ�ΪCaCO3��Mg��OH��2��д������CaCO3�����ӷ���ʽ ��
�۵�ˮ�ij���Ϊa��b��c�е� ���ڣ�
��2����ͼ2�ǹ�ҵ������þ�����̣�
�ٸ��ﲽ���н��Ȼ�þ��ˮ�Ͼ���ת��Ϊ��ˮ�Ȼ�þ�IJ��������� ��
���������������У�ѭ��ʹ�õ������� ��
��������Ϊ�����˲����ֱ�Ӽ���Mg��OH��2��MgO���ٵ�����ڵ�MgO�ƽ���þ���������Ż��������̣���Ĺ۵��� ���ͬ�⡱��ͬ�⡱���������� ��
������һ����ı��أ�������Դ�Ŀ��������þ��й�����ǰ����ij�غ�ˮ��pH��7.5��8.6֮�䣬������Ҫ���ӵĺ������±���
| �ɷ� | Na+ | K+ | Ca2+ | Mg2+ | Cl- | SO 42- | HCO 3+ | ����/mg?L-1 | 9360 | 83 | 200 | 1100 | 16000 | 1200 | 118 |

�������ĵ缫��ӦʽΪ
�ڵ��һ��ʱ�䣬�����������ˮ������ɷ�ΪCaCO3��Mg��OH��2��д������CaCO3�����ӷ���ʽ
�۵�ˮ�ij���Ϊa��b��c�е�
��2����ͼ2�ǹ�ҵ������þ�����̣�
�ٸ��ﲽ���н��Ȼ�þ��ˮ�Ͼ���ת��Ϊ��ˮ�Ȼ�þ�IJ���������
���������������У�ѭ��ʹ�õ�������
��������Ϊ�����˲����ֱ�Ӽ���Mg��OH��2��MgO���ٵ�����ڵ�MgO�ƽ���þ���������Ż��������̣���Ĺ۵���
 ��2011?����ģ�⣩����ѧ�뼼����ģ��
��2011?����ģ�⣩����ѧ�뼼����ģ��
