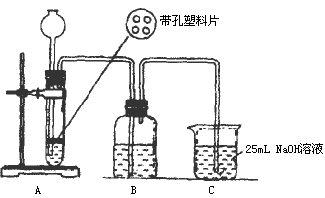
��Ŀ����
ijѧ������50mL NaOH��Һ����CO2���壬�Ʊ�Na2CO3��Һ��Ϊ�˷�ֹͨ���CO2�������������NaHCO3�������������ʵ�鲽�裺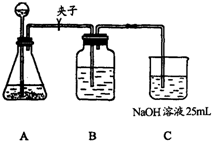
��ȡ25mL NaOH��Һ���չ�����CO2���壬��CO2���岻���ܽ⣻
��С�������Һ1��2�����ų���Һ���ܽ�Ķ�����̼���壻
���ڵõ�����Һ�м�����һ�루25mL��NaOH��Һ��ʹ��Һ��ֻ�ϣ�
��1�����ܷ��Ƶýϴ�����Na2CO3��
������
��������ƣ��ڢٲ�ʵ��װ����ͼ��ʾ��
��2��װ��Aʹ�õ��Լ���ʯ��ʯ��������Һ���ɷ�ʹ�ô������ʯ��ʯ��
��3��װ��Bʹ�õ��Լ���
��4��������Ϊʵ�鲽��ڢ۵�˳��Ե������Ȼ�ϣ�����У�������������Ϊ����Ϊʲô��

��ȡ25mL NaOH��Һ���չ�����CO2���壬��CO2���岻���ܽ⣻
��С�������Һ1��2�����ų���Һ���ܽ�Ķ�����̼���壻
���ڵõ�����Һ�м�����һ�루25mL��NaOH��Һ��ʹ��Һ��ֻ�ϣ�
��1�����ܷ��Ƶýϴ�����Na2CO3��
��
��
��������
NaHCO3+NaOH=Na2CO3+H2O����ǡ����ȫ��Ӧ��
NaHCO3+NaOH=Na2CO3+H2O����ǡ����ȫ��Ӧ��
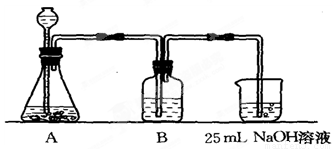
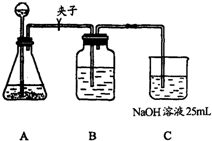
����������ƣ��ڢٲ�ʵ��װ����ͼ��ʾ��
��2��װ��Aʹ�õ��Լ���ʯ��ʯ��������Һ���ɷ�ʹ�ô������ʯ��ʯ��
����
����
��ԭ�������������ᷴӦ̫�죬������CO2������
���������ᷴӦ̫�죬������CO2������
����3��װ��Bʹ�õ��Լ���
����NaHCO3��Һ
����NaHCO3��Һ
����������HCl���壮
��HCl���壮
����4��������Ϊʵ�鲽��ڢ۵�˳��Ե������Ȼ�ϣ�����У�������������Ϊ����Ϊʲô��
���ԣ�������CO2����NaOH������ʹNaHCO3ȫ��ת��ΪNa2CO3
���ԣ�������CO2����NaOH������ʹNaHCO3ȫ��ת��ΪNa2CO3
����������1������̼����������������֮��Ĺ�ϵ������
��2������̼���Ƶ����ʷ�����
��3����������ijɷּ�ʵ��Ŀ�ķ�����
��4��������̼�����ʼ�̼�����ƺ��������Ƶ����ʵ���֮��Ĺ�ϵ������
��2������̼���Ƶ����ʷ�����
��3����������ijɷּ�ʵ��Ŀ�ķ�����
��4��������̼�����ʼ�̼�����ƺ��������Ƶ����ʵ���֮��Ĺ�ϵ������
����⣺��1���������ƺ���������̼��Ӧ����̼�����ƣ����ɵ�̼�����Ƶ����ʵ������������� �����ʵ�����ȣ���̼���������������Ʒ�Ӧǡ��1��1��Ӧ����̼���ƣ������ܣ�
�ʴ�Ϊ���ܣ�NaHCO3+NaOH=Na2CO3+H2O����ǡ����ȫ��Ӧ��
��2��̼�����Ƿ�ĩ״�Ĺ��壬��ͬ������ʯ��ʯ��̼���ƣ�̼���ƵĽӴ���������Է�Ӧ���ʿ죬�����ڶ�����̼�����գ����Բ��ܣ�
�ʴ�Ϊ�����ܣ����������ᷴӦ̫�죬������CO2�����գ�
��3���÷�Ӧ�Ƿ��ȷ�Ӧ�����ŷ�Ӧ�Ľ��У���Һ���¶����ߣ�������лӷ��ԣ��������ɵĶ�����̼�к����Ȼ������壬Ϊ�˳�ȥ������̼�е��Ȼ��������ֲ������µ����ʣ��ñ��͵�̼��������Һ�����Ȼ��⣮
�ʴ�Ϊ�����͵�NaHCO3��Һ����ȥ�Ȼ������壮
��4�����ʵ�鲽��ڢ۵�˳��Ե�����Һ���ܽ��˲��ֶ�����̼��������̼���������Ʒ�Ӧ��ʹ�������Ƶ������٣����Һ��̼�����ƺ��������Ƶ����ʵ�������ȣ����Բ���ǡ����ȫ��Ӧ����̼���ƣ����Բ��ԣ�
�ʴ�Ϊ�����ԣ�������CO2����NaOH������ʹNaHCO3ȫ��ת��ΪNa2CO3��
�ʴ�Ϊ���ܣ�NaHCO3+NaOH=Na2CO3+H2O����ǡ����ȫ��Ӧ��
��2��̼�����Ƿ�ĩ״�Ĺ��壬��ͬ������ʯ��ʯ��̼���ƣ�̼���ƵĽӴ���������Է�Ӧ���ʿ죬�����ڶ�����̼�����գ����Բ��ܣ�
�ʴ�Ϊ�����ܣ����������ᷴӦ̫�죬������CO2�����գ�
��3���÷�Ӧ�Ƿ��ȷ�Ӧ�����ŷ�Ӧ�Ľ��У���Һ���¶����ߣ�������лӷ��ԣ��������ɵĶ�����̼�к����Ȼ������壬Ϊ�˳�ȥ������̼�е��Ȼ��������ֲ������µ����ʣ��ñ��͵�̼��������Һ�����Ȼ��⣮
�ʴ�Ϊ�����͵�NaHCO3��Һ����ȥ�Ȼ������壮
��4�����ʵ�鲽��ڢ۵�˳��Ե�����Һ���ܽ��˲��ֶ�����̼��������̼���������Ʒ�Ӧ��ʹ�������Ƶ������٣����Һ��̼�����ƺ��������Ƶ����ʵ�������ȣ����Բ���ǡ����ȫ��Ӧ����̼���ƣ����Բ��ԣ�
�ʴ�Ϊ�����ԣ�������CO2����NaOH������ʹNaHCO3ȫ��ת��ΪNa2CO3��
���������⿼�����Ƶ���Ҫ����������ʣ�Ҫע����ǣ���ȥ������̼�е��Ȼ������岻���ñ��͵�̼������Һ����Ȼ�Ȼ����̼���Ʒ�Ӧ����������̼��̼���Ʒ�Ӧ���ɣ������˶�����̼���������Բ��ñ��͵�̼������Һ���ն�����̼�е��Ȼ������壮

��ϰ��ϵ�д�
�����Ŀ