��Ŀ����
����Ŀ��ij�о���ѧϰС��Ϊ�˲ⶨijƷ�����Ͻ������ĺ����������������ʵ�飺
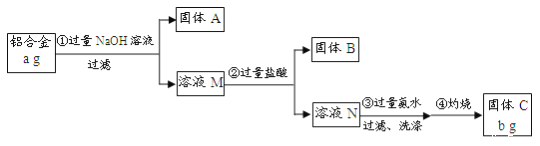
��֪�������Ͻ����Ҫ�ɷ�ΪAl2O3��MgO��CuO��SiO2��2NaOH+SiO2=Na2SiO3+H2O��Na2SiO3+
2HCl=2NaCl+H2SiO3����
��ش��������⣺
��1������A�ijɷ���_______��
��2������������ɳ��������ӷ���ʽΪ_____��
��3�����鲽����г����Ƿ�ϴ�Ӹɾ���ʵ�����Ϊ______��
��4������Ʒ����������������______������a��b��ʾ��
���𰸡�MgO��CuO Al3++3NH3��H2O=Al(OH)3��+3NH ȡ���һ��ϴ��Һ������AgNO3��Һ���������ɫ��������û��ϴ�Ӹɾ�����֮��ϴ�Ӹɾ� ![]() ��100%
��100%
��������
���Ͻ����Ҫ�ɷ�ΪAl2O3��MgO��CuO��SiO2����������NaOH��Ӧ����Al2O3��SiO2�����Թ���AΪMgO��CuO����ҺMΪNa2SiO3��NaAlO2��������ʱ����Na2SiO3+2HCl=2NaCl+H2SiO3����NaAlO2+4HCl=NaCl+AlCl3+2H2O�����Թ���BΪH2SiO3����ҺNΪAlCl3��HCl�����백ˮ��������������ͨ�����ˡ�ϴ�ӡ����յõ�Al2O3��
(1)���з�����֪��AΪMgO��CuO���ʴ�Ϊ��MgO��CuO��
(2)���������Al3+�백ˮ��Ӧ���ʴ�Ϊ��Al3++3NH3��H2O=Al(OH)3��+3NH��
(3)�����鲽����г����Ƿ�ϴ�Ӹɾ������������ӣ��ʴ�Ϊ��ȡ���һ��ϴ��Һ������AgNO3��Һ���������ɫ��������û��ϴ�Ӹɾ�����֮��ϴ�Ӹɾ���
(4)������Ԫ���غ��֪����������Ϊ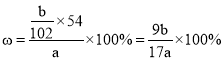 ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��100%��
��100%��

����Ŀ����a��b��c��d�ĸ������缫���йص�ʵ��װ�ü�����ʵ���������£�
ʵ��װ �� |
|
|
|
|
����ʵ������ | a��������С��b���������� | b�������������c���ޱ仯 | d���ܽ⣻c����������� | ������a������d�� |
�ɴ˿��ж������ֽ����Ļ��˳����(����)
A.a>b>c>dB.b>c>d>aC.d>a>b>cD.a>b>d>c



