��Ŀ����
4��������������Ҫ������������ԭ��������ˮ��Һ�ֳ�Ϊ˫��ˮ��������������ɱ����Ư�ȣ�ij��ѧ��ȤС��ȡһ�����Ĺ���������Һ��ȷ�ⶨ��������ĺ���������д���пհף�
��1����ȡ10.00mL�ܶ�Ϊ��g/mL�Ĺ���������Һ��250mL����ƿ�����������ƣ��У���ˮϡ�����̶ȣ�ҡ�ȣ���ȡϡ�ͺ�Ĺ���������Һ25.00mL����ƿ�У�����ϡ�����ữ��������ˮϡ�ͣ�������������
��2���ø�����ر���Һ�ζ������������䷴Ӧ�����ӷ���ʽ���£��뽫������ʵĻ�ѧ����������ѧʽ��д�ڷ����
��MnO${\;}_{4}^{-}$+��H2O2+��H+�T��Mn2++��H2O+����
��3���ζ�ʱ����������ر���Һע����ʽ�����ʽ����ʽ�����ζ����У��ζ������յ�������ǵ���һ�θ��������Һ����Һ��dz��ɫ����30���ڲ���ɫ��
��4���ظ��ζ����Σ�ƽ������cmol/L KMnO4����ҺVmL����ԭ����������Һ�й����������������Ϊ$\frac{0.085cV}{��}$��100%��
��5�����������ƫ�ߡ���ƫ�͡����䡱��
���ζ�ǰ�ζ��ܼ����������ݣ��ζ���������ʧ����ⶨ���ƫ��
�۲���ʽ�ζ���Һ��ʱ����ʼ���ӣ��ζ��յ�ƽ�ӣ���ζ����ƫ�ߣ�
������ƿ�ô���Һ��ϴ��Ȼ���ټ���25.00mL����Һ����ζ����ƫ�ߣ�
���� ��1������ȷ����һ�����һ�����ʵ���Ũ����Һ�õ����������ش�
��2����ȱ����ʽ��ƽ����ȷ��ȱʲô�����ݵ���ת���غ㣬��ƽ��ѧ����ʽ��
��3����ʽ�ζ��ܿ�����ȡ����ǿ�����Ե���Һ��������ؾ�����ɫ����������ɫ��
��4�����ݻ�ѧ����ʽ�������Ĺ����������Ȼ���������������
��5���ζ�ʵ�����������������ı���Һ������仯�жϲ������Ľ����
c�����⣩=$\frac{c������Һ��V������Һ��}{V��������Һ��}$��������Һ�������Խ�ⶨ���Խ�ߣ�
���ζ�ǰ�ζ��ܼ����������ݣ��ζ���������ʧ������һ������Һռ�������ݵ������
�۲���ʽ�ζ���Һ��ʱ����ʼ���ӣ��ζ��յ�ƽ�ӣ����ı���Һ�������
������ƿ�ô���Һ��ϴ��Ȼ���ټ���25.00mL����Һ�����ı���Һ���������
��� �⣺��1��ȷ����һ�����һ�����ʵ���Ũ����Һ������ƿ���ʴ�Ϊ������ƿ��
��2������ʽ�У����������ǿ�����ԣ��ܽ�˫��ˮ����Ϊ��������ȷ��ȱ����O2����Ԫ�ػ��ϼ۽�����5�ۣ�����1mol����ʱ����Ԫ�ػ��ϼ�����2�ۣ����ݵ���ת���غ㣬��ƽ��ѧ����ʽ�������ǰ���ϵ��Ϊ2��˫��ˮǰ���ϵ��Ϊ5������ԭ���غ�����ƽ��������ǰ���ϵ����
�ʴ�Ϊ��2��5��6��2��8��502��
��3�����ڸ�����ر���Һ����ǿ�����ԣ�����ֻ��ʹ����ʽ�ζ��ܣ��ζ������յ�������ǣ�����һ�θ��������Һ����Һ��dz��ɫ����30���ڲ���ɫ��
�ʴ�Ϊ����ʽ������һ�θ��������Һ����Һ��dz��ɫ����30���ڲ���ɫ��
��4�����ݻ�ѧ����ʽ���Եõ���ϵʽ��2MnO4-��5H2O2������c mol/L KMnO4����ҺV mL����cV��10-3mol�ĸ������ʱ������˫��ˮ�����ʵ�����2.5cV��10-3mol����ԭ�������������Ϊ��0.025cVmol��34g/mol=0.85cV������������Һ�й����������������Ϊ��$\frac{0.85cV}{10.00ml����g/mol}$��100%=$\frac{0.085cV}{��}$��100%��
�ʴ�Ϊ��$\frac{0.085cV}{��}$��100%��
��5�����ζ�ǰ�ζ��ܼ����������ݣ��ζ���������ʧ����һ������Һռ�������ݵ��������û�е�����ƿ����ⶨ���ƫ�ߣ�
�۲���ʽ�ζ���Һ��ʱ����ʼ���ӣ��ζ��յ�ƽ�ӣ����ı���Һ������ⶨ���ƫ�ߣ�
������ƿ�ô���Һ��ϴ��Ȼ���ټ���25.00mL����Һ�����ı���Һ��������ⶨ���ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�ƫ�ߣ�ƫ�ߣ�
���� ������һ���ۺ�֪ʶ��Ŀ������Ƕȹ㣬Ҫ��ѧ�����з����ͽ��������������Ѷȴ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | 15 g����-CH3�������еĵ�������NA | |
| B�� | 0.5 mol 1��3-����ϩ�����к���C�TC����ΪNA | |
| C�� | ��״���£�1 L������ȼ�պ����ɵ���̬����ķ�����Ϊ$\frac{5}{22.4}$NA | |
| D�� | ���³�ѹ�£�1 mol���������еĹ��ۼ���ĿΪ12NA |
| A�� | CO2��ˮ��Һ��c��H+����c��HCO3-����2c��CO32-�� | |
| B�� | ��Ũ�ȵ�HCN��Һ��Na0H��Һ�������ϣ�������ҺpH��7������Һ������Ũ�ȣ�c��Na+����c��CN-����c��OH-����c��H+�� | |
| C�� | 0.4mol•L-1ijһԪ��HA��Һ��0.2mol•L-1Na0H��Һ�������ϵ���Һ�У�2c��OH-��+c��A-��=2c��H+��+c��HA�� | |
| D�� | ��������HX��HY��Ϻ���Һ�е�c��H+��Ϊ��KaΪ����ƽ�ⳣ������c��H+��=$\frac{{K}_{a}��HX��•c��HX��}{c��{X}^{-}��}$+$\frac{{K}_{a}��HY��•c��HY��}{c��{Y}^{-}��}$+c��OH-�� |
��1�����о�������230�桫270��ʱ�ϳ���Ϊ������Ϊ̽Ѱ�ϳ�������ʵ���ʼ��ɱȣ��ֱ���230�桢250���270��ʱ����ʵ�飬ʵ������ͼ��230���ʵ��������Ӧ��������X������ĸ���� �����COת���ʵĽǶȵ��ۺϷ��������¶��¹�ҵ�������˲��õĺϳ������n��H2����n��CO���ı�ֵ��Χ��B������ĸ����
A��1��1.5��������B��2.5��3C��3.5��4.5

��2���Ƽ״�����Ҫ����������ͨ�����з�Ӧ��ȡ��H2O��g��+CO��g��?H2��g��+CO2��g������H��0��ij�¶��¸÷�Ӧ��ƽ�ⳣ��K=1���ش��������⣺
�ٸ��¶��£�����ʼʱc��CO��=2mol•L-1��c��H2O��=3mol•L-1����Ӧ����һ��ʱ����CO��Ũ��Ϊ1mol•L-1�����ʱ�÷�Ӧv��������v���棩�����������������=������
���������¶ȣ��÷�Ӧ��Kֵ���������������С�����䡱����
��3���״���һ�ֻ���ԭ�ϣ���ҵ�Ϻϳɼ״��ķ�Ӧ��CO��g��+2H2��g��?CH3OH��g����H=-90.8kJ•mol-1��
�����¶ȡ��ݻ���ͬ��3���ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й��������£�
| ���� | �� | �� | �� |
| ��Ӧ��Ͷ���� | 1molCO��2molH2 | 1mol CH3OH | 2molCO��4molH2 |
| CH3OH��Ũ�ȣ�mol/L�� | c1 | c2 | c3 |
| ��Ӧ�������仯 | �ų�Q1 kJ | ����Q2 kJ | �ų�Q3 kJ |
�ڱ仯��������ֵQ�У�Q1 ��Q2�ĺ���90.8���������ֵ����
��4��Ŀǰ���Լ״�Ϊԭ�ϵ�ȼ�ϵ���Ѿ�Ӧ���ڹ�ҵ��������ͼ�Ǽ״�ȼ�ϵ��Ӧ�õ�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH�T2K2CO3+6H2O��
 ��
���������ͼ��д���пհף�
�ҳ���A�缫�ĵ缫��ӦʽΪAg++e-=Ag���׳���ͨ��CH3OH�缫�ĵ缫��ӦʽΪCH3OH-6e-+8OH-=CO32-+6H2O��
| A�� | Ũ�������ǿ�����ԣ������������ۻ�������A1��Fe���������桢����Ũ���� | |
| B�� | �������а뵼�����ʣ��������������ά | |
| C�� | ����������Ư���ԣ������ڼӹ�ʳƷʹʳƷ���� | |
| D�� | Fe3+���������ԣ������ھ�ˮ |
| A�� | 1 mol CH3+��̼�����ӣ��к���������Ϊ8NA | |
| B�� | ��״���£�22.4 L�嵥������ԭ����ĿΪ2NA | |
| C�� | �����£�100mLlmol��L-l������4.6 g�Ʒ�Ӧ����H2������ĿΪ0.1 NA | |
| D�� | �ܱ�����ʢ��0.1 mol N2��0.3 mol H2��һ�������³�ַ�Ӧ��ת�Ƶ��ӵ���ĿΪ0.6 NA |
| A�� | ��ʯ�ҡ����ס���ʯ�� | B�� | �ɱ��������Ȼ��� | ||
| C�� | ��Ȼ�������������� | D�� | �ռҺ̬������� |
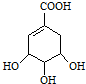 ���л�������У���̼ԭ�������ĸ���ͬ��ԭ�ӻ�ԭ���ţ���̼ԭ�ӳ�Ϊ���Գ�̼ԭ�ӣ�������̼ԭ�ӣ�����������̼ԭ�ӵ��л�����й�ѧ���ԣ��˽����ڲݱ�ֲ���Ҫ�����й���Խ�ϣ����ҹ���䳣������������ϣ�����ҽѧ�о��ɹ���ʾ���Ӱ˽��п�����ȡ��ç���ᣬ����һ�ְ�ɫ���壬����ˮ������ȡ��������ҩ�Ļ���ԭ�ϣ�ç����Ľṹʽ��ͼ��
���л�������У���̼ԭ�������ĸ���ͬ��ԭ�ӻ�ԭ���ţ���̼ԭ�ӳ�Ϊ���Գ�̼ԭ�ӣ�������̼ԭ�ӣ�����������̼ԭ�ӵ��л�����й�ѧ���ԣ��˽����ڲݱ�ֲ���Ҫ�����й���Խ�ϣ����ҹ���䳣������������ϣ�����ҽѧ�о��ɹ���ʾ���Ӱ˽��п�����ȡ��ç���ᣬ����һ�ְ�ɫ���壬����ˮ������ȡ��������ҩ�Ļ���ԭ�ϣ�ç����Ľṹʽ��ͼ�� $��_{��}^{Ũ����}$
$��_{��}^{Ũ����}$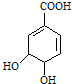 +H2O��
+H2O��