��Ŀ����
��֪A��B��C��D��E��F����Ԫ�ص�ԭ���������ε����� Aλ�����ڱ���s������ԭ���е��Ӳ�����δ�ɶԵ�������ͬ��B�Ļ�̬ԭ���е���ռ������������ͬ��ԭ�ӹ������ÿ�ֹ���еĵ���������ͬ��Dԭ�ӵĺ���ɶԵ�������δ�ɶԵ�������3��;A��E��ԭ���������10��F+��M�����ȫ������
��ش��������⣺������ʱ��A��B��C��D��E��F������Ӧ��Ԫ�ط��ű�ʾ��
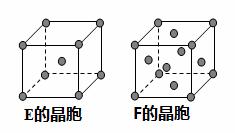
��1��C�Ļ�̬ԭ�ӵ���Χ�����Ų�ʽΪ ,���ڱ���F
���� ����
��2��C��D��E��Ԫ�صĵ縺���ɴ�С��˳��Ϊ�� �������Ӱ뾶�ɴ�С��˳��Ϊ�� ��
��3��BD�����У�Bԭ����Dԭ�Ӷ��ﵽ8�����ȶ��ṹ����BD�ĵ���ʽ�� ��
��4��BD2��EDA��Ӧʱ��ͨ�����Ʒ�Ӧ������ʵ���֮�ȣ����Եõ���ͬ�IJ����ͬ�����£���ˮ���ܽ�Ƚ�С�IJ����� ��д��ѧʽ������ԭ���Ǹû����������Ӽ���γɶ������ӻ�����״���ӡ��û������������ܹ���ϵ�ԭ���ǣ� ��
 ��5��B2D42���� �����ӣ��ǵȵ����壬B2D42�����Ӿ��н�ǿ�Ļ�ԭ�ԣ�����ʹ����KMnO4��Һ��ɫ(��ԭ����ΪMn2+)��д���仯�����ӷ���ʽ�� ��
��5��B2D42���� �����ӣ��ǵȵ����壬B2D42�����Ӿ��н�ǿ�Ļ�ԭ�ԣ�����ʹ����KMnO4��Һ��ɫ(��ԭ����ΪMn2+)��д���仯�����ӷ���ʽ�� ��
��6����ͼ�ֱ���E��F�ľ�����������E��F����λ��֮��Ϊ ��
��7����ͬ�����£�V1mLBD2��V2mLCD�Ļ������ͨ������E2D2��������������Ϊ(V1+V2)/2mL��������CD��E2D2�ķ�Ӧ��,��V1��V2�Ĺ�ϵ���㣺 ��
(1)2s22P 3 ds
(2)O��N��Na N 3�� �� O2����Na��
(3) ![]()
(4)NaHCO3 HCO3������֮��������
��5��N2O4 5C2O42��+2MnO4��+16H+=10CO2��+2Mn2++8H2O
(6)2:3
��7��V1��V2

 ��У����ϵ�д�
��У����ϵ�д�| A��Fe��Cu��Al��Ag | B��Al��Cu��Fe��Ag | C��Cu��Ag��Al��Fe | D��Ag��Al��Cu��Fe |

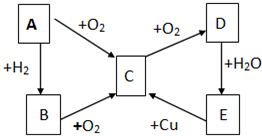 ��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ� ����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�