��Ŀ����
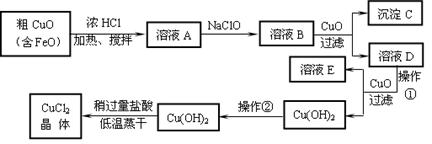 |
��ҵ����ȡCuCl2�������������£�
�����±����ݣ��ش����⣺
| �� �� | Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
|
| 8.0��10��16 | 2.2��10��20 | 4.0��10��38 |
| ��ȫ����ʱ��pH��Χ | ��9.6 | ��6.4 | 3��4 |
��1��д���������ӷ���ʽ��
A��B �� B��C+D ��
��2����ҺA�м���NaClO��Ŀ���� ��
��3������ҺB�м���CuO�������� ��
��4��������Ӧ���Ƶ�pH��Χ�ǣ� �������ڵ�Ŀ���� ��
��5����Cu(OH)2��������ʹCu(OH)2ת��ΪCuCl2�����á��Զ������ᡱ�͡��������ɡ���Ŀ���� ��
��1��2Fe2++ClO��+2H+ = 2Fe3++Cl��+H2O ��2�֣� CuO+2H+ = Cu2++H2O ��2�֣�
��2����Fe2������ΪFe3����ʹ��һ������Fe(OH)3������ ��3�֣�
��3��������Һ��pHΪ3��4����4��pH��6.4����ʹFe3����ȫת��ΪFe(OH)3��������ȥFe3+ ��3�֣�
��4�����ڻ����6.4�����6.4�� ��2�֣� ϴ��Cu(OH)2����Ŀ��������� ��2�֣�
��5������Cu2����ˮ�⣬��ֹCuCl2�����к���Cu(OH)??2���� ��3�֣�
����:

 ��������ϵ�д�
��������ϵ�д���10�֣���ҵ����ȡCuCl2�������������£�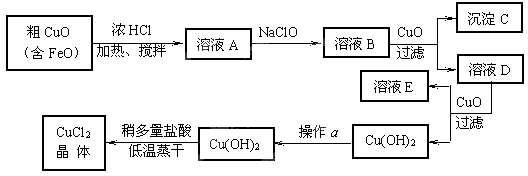
�����±����ݣ��ش��������⣺
| �� �� | Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
| ��ȫ����ʱ��pH��Χ | ��9.6 | ��6.4 | 3��4 |
�˷�Ӧ���ӷ���ʽΪ ��
�� ����ҺB�м���CuO�������� ��
�� ����a��Ŀ���� ��
�� ��Cu(OH)2��������ʹCu(OH)2ת��ΪCuCl2�����ö�������͵������ɵ�Ŀ���� ��
��10�֣���ҵ����ȡCuCl2�������������£�
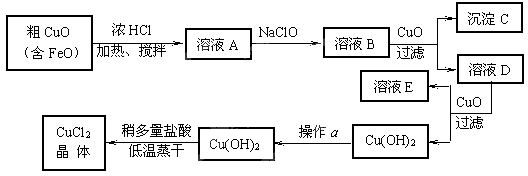
�����±����ݣ��ش��������⣺
|
�� �� |
Fe(OH)2 |
Cu(OH)2 |
Fe(OH)3 |
|
��ȫ����ʱ��pH��Χ |
��9.6 |
��6.4 |
3��4 |
�� ��ҺA�м���NaClO��Ŀ���� ��
�˷�Ӧ���ӷ���ʽΪ ��
�� ����ҺB�м���CuO�������� ��
�� ����a��Ŀ���� ��[��Դ:]
�� ��Cu(OH)2��������ʹCu(OH)2ת��ΪCuCl2�����ö�������͵������ɵ�Ŀ���� ��
��8��,ÿ��2�֣���ҵ����ȡCuCl2�������������£�
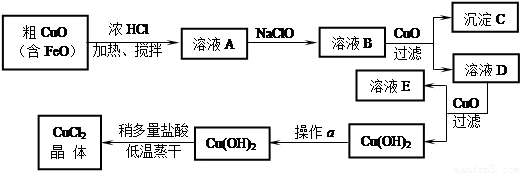
�����±����ݣ��ش��������⣺
|
�� �� |
Fe(OH)2 |
Cu(OH)2 |
Fe(OH)3 |
|
�ܶȻ���25�� |
8.0��10��16 |
2.2��10��20 |
4.0��10��38 |
|
��ȫ����ʱ��pH��Χ |
��9.6 |
��6.4 |
3��4 |
�� ��ҺA�м���NaClO��Ŀ���� ��
�� ����ҺB�м���CuO�������� ��
�� ����a��Ŀ���� ��
�� ��Cu(OH)2��������ʹCu(OH)2ת��ΪCuCl2�����ö�������͵������ɵ�Ŀ���� ��

