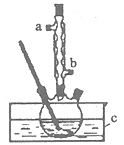
题目内容
【题目】ClO2是一种优良的消毒剂,浓度过高时易发生分解,常将其制备成NaClO2固体以便运输和贮存。过氧化氢法制备NaClO2固体的实验装置如图1所示。
已知:2NaClO2+H2O2+H2SO4=2ClO2↑+O2↑+Na2SO4+2H2O
2ClO2+H2O2+2NaOH=2NaClO2+O2↑+2H2O
ClO2熔点-59℃、沸点11℃;H2O2沸点150℃

请回答:
⑴仪器A的作用是_____;冰水浴冷却的目的是_____(写出两种)。
⑵空气流速过快或过慢,均降低NaClO2产率,试解释其原因______。
(3)Cl-存在时会催化ClO2的生成。反应开始时在三颈烧瓶中加入少量盐酸,ClO2的生成速率大大提高,并产生微量氯气。该过程可能经两步反应完成,将其补充完整:①_____(用离子方程式表示),②H2O2+Cl2=2Cl-+O2+2H+。
(4) H2O2浓度对反应速率有影响。通过图2所示装置将少量30% H2O2溶液浓缩至40%,B处应增加一个设备。该设备的作用是______,馏出物是_______。

(5)抽滤法分离NaClO2过程中,下列操作不正确的是_______
A.为防止滤纸被腐蚀,用玻璃纤维代替滤纸进行抽滤
B.先转移溶液至漏斗,待溶液快流尽时再转移沉淀
C.洗涤沉淀时,应使洗涤剂快速通过沉淀
D.抽滤完毕,断开水泵与吸滤瓶间的橡皮管后,关闭水龙头
【答案】 防倒吸 防止H2O2分解,使ClO2液化,促进ClO2的吸收 过慢会导致反应的三颈烧瓶内ClO2浓度过高,发生分解,产率降低;过快则反应物反应不充分 2ClO3-+2Cl-+4H+=Cl2+2ClO2+2H2O 减少体系内压强,降低H2O的沸点(促进H2O蒸出,同时减少H2O2的受热分解等合理答案亦可) H2O C
【解析】(1)仪器A是安全瓶,作用是防倒吸;双氧水易分解,ClO2的熔点降低,因此冰水浴冷却的目的是防止H2O2分解,使ClO2液化,促进ClO2的吸收。(2)如果空气流速过慢会导致反应的三颈烧瓶内ClO2浓度过高,发生分解,产率降低;空气流速过快则反应物反应不充分,原料利用率不高。(3)Cl-存在时会催化ClO2的生成,根据第二步反应可知产生氯离子,因此第一步是消耗氯离子,离子方程式为2ClO3-+2Cl-+4H+=Cl2+2ClO2+2H2O。(4)由于是浓缩双氧水,则馏出物是水,而双氧水易分解,因此该设备的作用是减少体系内压强,降低H2O的沸点(促进H2O蒸出,同时减少H2O2的受热分解等)。(5)A.为防止滤纸被腐蚀,用玻璃纤维代替滤纸进行抽滤,A正确;B.先转移溶液至漏斗,待溶液快流尽时再转移沉淀,B正确;C.洗涤沉淀时,应将少量溶剂洒到固体上,静置片刻,再将其抽干,C错误;D.抽滤完毕,为防止水倒吸到抽滤瓶内,应断开水泵与吸滤瓶间的橡皮管后,关闭水龙头,D正确,答案选C。

 一课一练课时达标系列答案
一课一练课时达标系列答案【题目】只给出下列甲中和乙中对应的量,不能组成一个求物质的量浓度公式的是
序号 | ① | ② | ③ | ④ | ⑤ |
甲 | 物质微粒数 | 标准状况下 气体摩尔体积 | 溶质Na2SO4的质量 | 溶质KCl的 质量分数 | 非标准状况 下物质的质量 |
乙 | 阿伏加德罗常数 | 标准状况下 气体密度 | 溶液的体积 | 溶液的密度 | 物质的摩尔质量 |
A. ①②⑤ B. ①②④⑤ C. ③④ D. ③