��Ŀ����
��֪���ȼ��a g��Ȳ����ʱ����1mol������̼�����Һ̬ˮ�����ų�����b kJ������Ȳȼ�յ��Ȼ�ѧ����ʽ��ȷ����
A. 2C2H2(g)��5O2(g)��4CO2(g)��2H2O(l)�� ��H����2b kJ??mol??1
B. C2H2(g)��5/2O2(g)��2CO2(g)��H2O(l)�� ��H��2b kJ??mol??1
C. 2C2H2(g)��5O2(g)��4CO2(g)��2H2O(l)�� ��H����4b kJ??mol??1
D. 2C2H2(g)��5O2(g)��4CO2(g)��2H2O(l)�� ��H��b kJ??mol??1
C
����:
�ʱ�Ϊ��-��ֵ��1mol��ȲӦ������2mol������̼�����Һ̬ˮ���ų�����2b kJ.

��ϰ��ϵ�д�
�����Ŀ
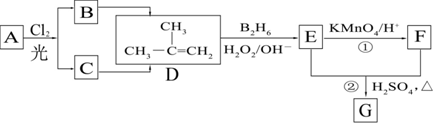
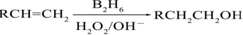 ��B2H6Ϊ�����飩
��B2H6Ϊ�����飩
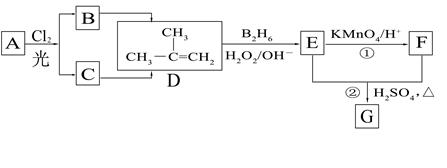
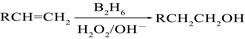 ��B2H6Ϊ�����飩
��B2H6Ϊ�����飩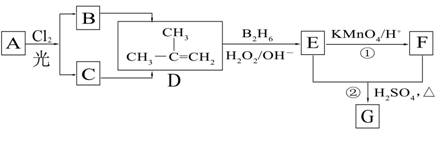
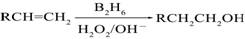 ��B2H6Ϊ�����飩
��B2H6Ϊ�����飩