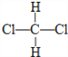
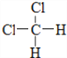
��Ŀ����
����Ŀ��ij��ѧʵ������Ҫ0.2mol/L NaOH��Һ500mL��0.5mol/L������Һ450mL��������������Һ����������ش��������⣺
��1����ͼ��ʾ��������������Һ�϶�����Ҫ����������ţ�������������Һ�����õ��IJ��������������������ƣ��� 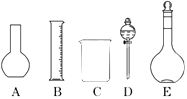
��2������ƿ��������Һ����Ҫ����������ƿ�ϱ������������е�����д��ţ���
���¶� ��Ũ�� ������ ��ѹǿ ����ʽ���ʽ �̶���
��3������ʱ������ȷ�IJ���˳���ǣ�����ĸ��ʾ��ÿ����ĸֻ����һ�Σ� ��
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ
B��ȷ��ȡ���������������ƹ������ձ��У��ټ�������ˮ��Լ50mL�����ò���������������ʹ�����ܽ⣬��ȴ������
C��������ƿ�ǽ���ҡ��
D�����ܽ������������Һ�ز�����ע������ƿ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ��Һ��ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�2��3cm��
��4�����ݼ��㣬����0.2mol/L NaOH��Һ500mL�� NaOH���������Ϊ��g��
��5�����ƹ������������ձ��н�Ũ�������ϡ�ͣ�ϡ��ʱ���������ǣ� ��
��6���������Ƶ�ϡH2SO4���вⶨ������ʵ��Ũ��С��0.5mol/L���������������Щ��������������Ũ��ƫС����д��ĸ�� ��
A.����Ͳ��ȡŨ����ʱ��������Ͳ�Ŀ̶�
B.����ƿδ���T����������Һ
C.Ũ�������ձ���ϡ�ͺ�δ��ȴ������ת�Ƶ�����ƿ�У������ж���
D.������ƿת��ʱ��������Һ�彦��
E.������ƿ�ж���ʱ��������ƿ�̶���
F.�ձ�δ����ϴ��
G.���ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶��ߣ�
���𰸡�
��1��AD������������ͷ�ι�
��2���٢ۢ�
��3��B��D��A��F��E��C
��4��4.0
��5����Ũ���������ձ��ڻ���ע��ˮ�У�ͬʱ�����ò���������
��6��BFG
���������⣺��1��������Һ�IJ������裺���ȼ������Ҫ�����ʵ�������Ũ�����������Ȼ����ƽ��������Ͳ��ȡ����������ձ����ܽ⣨ϡ�ͣ���ͬʱ�ò��������裬����Һ��ȴ�����º��ò�����������Һ��500ml����ƿ��Ȼ��ϴ���ձ��Ͳ�����2��3�Σ���ϴ��ҺҲע������ƿ��Ȼ��������ƿ��עˮ����Һ����̶���1��2CMʱ�����ý�ͷ�ι���μ��룬����Һ����̶������У�Ȼ��ҡ�ȡ�װƿ���ڴ˹������õ��������У���ƽ����Ͳ���ձ�����������500ml����ƿ����ͷ�ιܣ���ȱ�ٵ������У���ͷ�ιܡ�������������Ҫ���ǣ�ƽ����ƿ�ͷ�Һ©�������Դ��ǣ�A��D������������ͷ�ιܣ���2������ƿΪ����һ�����ʵ���Ũ����Һר������������ƿ�ϱ��������¶ȡ��������̶��ߣ�
��ѡ���٢ۢޣ���3������һ�����ʵ���Ũ����Һ�IJ��裺���㡢������ϡ�͡���ȴ����Һ�����ݡ�ҡ�ȡ�װƿ�ȣ�������ȷ��˳��Ϊ��BDAFEC��
���Դ��ǣ�BDAFEC����4������0.2mol/L NaOH��Һ500mL�� NaOH���������Ϊ��0.2mol/L��40g/mol��0.5L=4.0g��
���Դ��ǣ�4.0����5��Ũ����ϡ�Ͳ����������ȣ�ϡ�͵���ȷ����Ϊ����Ũ���������ձ��ڻ���ע��ˮ�У�ͬʱ�����ò��������裻
���Դ��ǣ���Ũ���������ձ��ڻ���ע��ˮ�У�ͬʱ�����ò��������裻��6��A������Ͳ��ȡŨ����ʱ��������Ͳ�Ŀ̶ȣ�������ȡ��Ũ�������ƫ�����ʵ����ʵ���ƫ����ҺŨ��ƫ�ߣ���A��ѡ��
B������ƿδ���T����������Һ������Һ��������ʵ���������Ӱ�죬��ҺŨ�Ȳ��䣬��B��ѡ��
C��Ũ�������ձ���ϡ�ͺ�δ��ȴ������ת�Ƶ�����ƿ�У������ж��ݣ���ȴ����Һ���ƫС����ҺŨ��ƫ�ߣ���C��ѡ��
D��������ƿת��ʱ��������Һ�彦�����������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���Dѡ��
E��������ƿ�ж���ʱ��������ƿ�̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���E��ѡ��
F���ձ�δ����ϴ�ӣ��������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���Fѡ��
G�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ���Gѡ��
��ѡ��BFG��
�����㾫����������Ҫ����������һ�����ʵ���Ũ�ȵ���Һ�����֪ʶ�㣬��Ҫ�����������ʵ���Ũ����Һʱ�������ձ�������ˮ������ƿ�̶���1cm��2cm���ٸ��ý�Ͷ�ιܼ�ˮ���̶��߲�����ȷ�����⣮

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�