��Ŀ����
��0.1molMg��Al���������100mL3mol/LHCl�У��ٵμ�1mol/LNaOH��Һ���ڵμ�NaOH��Һ�Ĺ����У�����������m��NaOH��Һ���V�仯��ͼ��ʾ��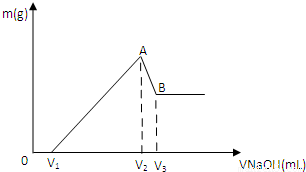
��1��д��A��B�η�Ӧ�����ӷ���ʽ______��
��2��A������������ʵ���n=______��
��3����ʹMg2+��Al3+�պó�����ȫ�������NaOH��Һ�����V2=______mL��
��4����V1=60mL����������Mg�����ʵ���n��Mg��=______��V3������NaOH��Һ�����ΪV3=______
mL��
���𰸡���������1��A��B������������������������Һ�����ķ�Ӧ���ݴ�д�����ӷ���ʽ��
��2��A��ʱ���������˵��þ����ȫ�����ɳ���������ԭ���غ������������ʵ�����
��3����ʹMg2+��Al3+�պó�����ȫʱ����Һ�е��������Ȼ��ƣ�����������������������Ƶ������
��4����V1=60mL��˵��������ʣ�࣬�����������Ƶ�������ʣ������������Ӷ��ó��ͽ�����Ӧ��������������ݽ���������֮��Ĺ�ϵ����þ��������B��ʱ��Һ�е�������ƫ�����ƺ��Ȼ��ƣ�����ԭ���غ�����������Ƶ������
����⣺��1��A��B������������������������Һ�����ķ�Ӧ�����ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O���ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��2��A��ʱ���������˵��þ����ȫ�����ɳ���Mg��OH��2��Al��OH��3������ԭ���غ�֪A������������ʵ�������þ�������ʵ�����Ϊ0.1mol���ʴ�Ϊ��0.1mol��
��3����ʹMg2+��Al3+�պó�����ȫʱ����Һ�е��������Ȼ��ƣ�����������ʵ��������������Ƶ����ʵ���=3mol/L×0.1L=0.3mol���������Ƶ����=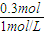 =300mL��
=300mL��
�ʴ�Ϊ��300��
��4����V1=60mL��˵��������ʣ�࣬ʣ����������ʵ���=1mol/L×0.06L=0.06mol����ͽ�����Ӧ����������ʵ���=3mol/L×0.1L-0.06mol=0.24mol��
��þ�����ʵ�����x���������ʵ�����y��
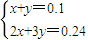
���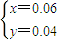 ��
��
����þ�����ʵ�����0.06mol��
B��ʱ��Һ�е�������ƫ�����ƺ��Ȼ��ƣ�����ԭ���غ�֪��n��NaOH��=n��Al��+n��HCl��=0.04mol+0.3mol=0.34mol�������������Ƶ����=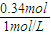 =340mL��
=340mL��
�ʴ�Ϊ��0.06mol��340��
���������⿼��þ���������ʼ�ͼ���������ȷ���߱仯��ʽ�ķ�Ӧ���յ�ʱ��Һ�е������ǽⱾ��ؼ���ע����ԭ���غ�������Ѷ��еȣ�
��2��A��ʱ���������˵��þ����ȫ�����ɳ���������ԭ���غ������������ʵ�����
��3����ʹMg2+��Al3+�պó�����ȫʱ����Һ�е��������Ȼ��ƣ�����������������������Ƶ������
��4����V1=60mL��˵��������ʣ�࣬�����������Ƶ�������ʣ������������Ӷ��ó��ͽ�����Ӧ��������������ݽ���������֮��Ĺ�ϵ����þ��������B��ʱ��Һ�е�������ƫ�����ƺ��Ȼ��ƣ�����ԭ���غ�����������Ƶ������
����⣺��1��A��B������������������������Һ�����ķ�Ӧ�����ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O���ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��2��A��ʱ���������˵��þ����ȫ�����ɳ���Mg��OH��2��Al��OH��3������ԭ���غ�֪A������������ʵ�������þ�������ʵ�����Ϊ0.1mol���ʴ�Ϊ��0.1mol��
��3����ʹMg2+��Al3+�պó�����ȫʱ����Һ�е��������Ȼ��ƣ�����������ʵ��������������Ƶ����ʵ���=3mol/L×0.1L=0.3mol���������Ƶ����=
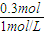 =300mL��
=300mL���ʴ�Ϊ��300��
��4����V1=60mL��˵��������ʣ�࣬ʣ����������ʵ���=1mol/L×0.06L=0.06mol����ͽ�����Ӧ����������ʵ���=3mol/L×0.1L-0.06mol=0.24mol��
��þ�����ʵ�����x���������ʵ�����y��
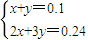
���
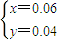 ��
������þ�����ʵ�����0.06mol��
B��ʱ��Һ�е�������ƫ�����ƺ��Ȼ��ƣ�����ԭ���غ�֪��n��NaOH��=n��Al��+n��HCl��=0.04mol+0.3mol=0.34mol�������������Ƶ����=
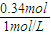 =340mL��
=340mL���ʴ�Ϊ��0.06mol��340��
���������⿼��þ���������ʼ�ͼ���������ȷ���߱仯��ʽ�ķ�Ӧ���յ�ʱ��Һ�е������ǽⱾ��ؼ���ע����ԭ���غ�������Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ