��Ŀ����
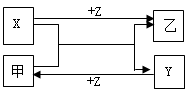
��16�֣��������й������ת���Ĺ�ϵͼ������A�׳����죬��Ϊǿ�ᣬ��Ϊ��ԭ�����壬��Ϊǿ����Һ��GΪ���ɫ������I����ɫ��dz��ɫ��
��1����F����Na+��SO42-��ɵ���Һ����Ļ�ѧʽ�� ���о�A���ʵ�һ����; ��
��2����D����ʹ����ʯ��ˮ����ǵ����壬���ҵĻ�ѧʽΪ �������ڵ��ʡ��ᡢ���е� ����I��Һ�м�������������Һ�����Թ۲쵽�������� ����Ӧ�����ӷ���ʽ�ͻ�ѧ����ʽ������ ��
��
��3��д��G A+C�Ļ�ѧ����ʽ�� ��
A+C�Ļ�ѧ����ʽ�� ��
��4����A�л�������Al2O3����ȥ���ʵķ����Ǽ�������� ���÷�Ӧ�����ӷ���ʽΪ ��
��5����E��A��ɵĻ������ϡH2SO4���ã�����ǡ���ܽ⣬������Һ�в���Fe3���������ɵ�Fe2����H2�����ʵ���֮��Ϊ4:1����Ӧ����Fe2O3��Fe��H2SO4�����ʵ���֮��Ϊ____ ____��

��1����F����Na+��SO42-��ɵ���Һ����Ļ�ѧʽ�� ���о�A���ʵ�һ����; ��
��2����D����ʹ����ʯ��ˮ����ǵ����壬���ҵĻ�ѧʽΪ �������ڵ��ʡ��ᡢ���е� ����I��Һ�м�������������Һ�����Թ۲쵽�������� ����Ӧ�����ӷ���ʽ�ͻ�ѧ����ʽ������ ��
��
��3���G
 A+C�Ļ�ѧ����ʽ�� ��
A+C�Ļ�ѧ����ʽ�� ����4����A�л�������Al2O3����ȥ���ʵķ����Ǽ�������� ���÷�Ӧ�����ӷ���ʽΪ ��
��5����E��A��ɵĻ������ϡH2SO4���ã�����ǡ���ܽ⣬������Һ�в���Fe3���������ɵ�Fe2����H2�����ʵ���֮��Ϊ4:1����Ӧ����Fe2O3��Fe��H2SO4�����ʵ���֮��Ϊ____ ____��
(16��)��1��H2SO4��������ɫ�����Ϳ��
��2��CO�� �
���ɰ�ɫ������Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ��
Fe2++2OH-=Fe(OH)2����4Fe(OH)2+O2+2H2O="4" Fe(OH)3��
��3��2Fe��OH��3 Fe2O3+3H2O
Fe2O3+3H2O
��4��NaOH��Һ��Al2O3+2OH-=2AlO2-+H2O
��5��1: 2:4��3�֣�
��2��CO�� �
���ɰ�ɫ������Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ��
Fe2++2OH-=Fe(OH)2����4Fe(OH)2+O2+2H2O="4" Fe(OH)3��
��3��2Fe��OH��3
 Fe2O3+3H2O
Fe2O3+3H2O ��4��NaOH��Һ��Al2O3+2OH-=2AlO2-+H2O
��5��1: 2:4��3�֣�
���������A�׳����죬����������������GΪ���ɫ��������������������I����ɫ��dz��ɫ�������������ӡ�
��1����FΪ�����ơ�GΪ���ɫ�����������������ݸ��ֽⷴӦ���ص㣬���Ʋ�B�����ֱ�Ϊ���������������ƣ����BΪ���������Ӧ��������жϼ�Ϊ���ᣬCΪˮ��
��2����D����ʹ����ʯ��ˮ����ǵ����壬���ж�����DΪ������̼������ӦA+�ҡ�D+E������AΪ����������Ϊ��ԭ�����壬��ΪCO��EΪ������������I����Һ��dz��ɫ�����ƶ�IΪ�������ӣ���Ϊһ���ᣬ���������������ȣ������������Ե����ᡣ������Ӧ���ɰ�ɫ������Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ������ʽΪFe2++2OH-=Fe(OH)2����4Fe(OH)2+O2+2H2O="4" Fe(OH)3��
��3�����������ڼ��������»�ֽ�Ϊ��������ˮ��2Fe��OH��3
 Fe2O3+3H2O
Fe2O3+3H2O��4����ȥ��������������һ���Ǽ�������������Һ����ֻ��������������������Ӧ��
��5������H2Ϊ1mol����Fe2��Ϊ4mol��
���ݷ���ʽ
Fe+H2SO4=FeSO4+H2��
1mol 1mol 1mol 1mol
������Ӧ������Fe2��Ϊ1mol������3mol Fe2�����������������ɣ�
Fe 2(SO4)3+ Fe= 3FeSO4
1mol 1mol 3mol
��֪Fe 2(SO4)3Ϊ1mol��FeΪ2mol��
Fe2O3+3H2SO4=Fe 2(SO4)3+3H2O��
1mol 3mol
������һ����4mol�����Ա�����1: 2:4
�����������ǵ�ת���������Ǹ��е�һ���ѵ㣬�����ۺϿ�����������֪ʶ��ͬʱ��ת�����漰�����㣬���ڽ��ѵ�һ���ۺ��⡣

��ϰ��ϵ�д�
�����Ŀ