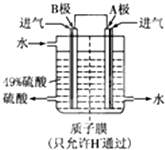
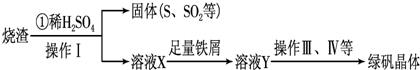
��Ŀ����
��2012?������ģ�����и��������ۺ������ǣ�������
|
������A�����������������Ȼ�泥�
B����Ӧ�����ԣ���Ӧ�������ᱵ�������ƺ�ˮ��
C������������ԭ��Ӧ�������ᱵ��NO��ˮ��
D����Ӧ����̼����ƺ�HClO��
B����Ӧ�����ԣ���Ӧ�������ᱵ�������ƺ�ˮ��
C������������ԭ��Ӧ�������ᱵ��NO��ˮ��
D����Ӧ����̼����ƺ�HClO��
����⣺A����AlCl3��Һ�е��������ˮ�����ӷ�ӦΪAl3++3NH3?H2O�TAl��OH��3��+NH4+�����ۺ�������A��ȷ��
B����������������������Ӧ�����Ե����ӷ�ӦΪSO42-+2H++Ba2++2OH-�T2H2O+BaSO4�������۴���B����
C�������ᱵ�ܽ���ϡ�����е����ӷ�ӦΪ3BaSO3+2NO3-+2H+�T3BaSO4��+H2O+2NO�������۴���C����
D������������̼ͨ����������Һ�е����ӷ�ӦΪClO-+H2O+CO2�THClO+HCO3-�����۴���D����
��ѡ��A��
B����������������������Ӧ�����Ե����ӷ�ӦΪSO42-+2H++Ba2++2OH-�T2H2O+BaSO4�������۴���B����
C�������ᱵ�ܽ���ϡ�����е����ӷ�ӦΪ3BaSO3+2NO3-+2H+�T3BaSO4��+H2O+2NO�������۴���C����
D������������̼ͨ����������Һ�е����ӷ�ӦΪClO-+H2O+CO2�THClO+HCO3-�����۴���D����
��ѡ��A��
���������⿼��ѧ�������ӷ���ʽ�������жϣ�������ȷ�����ۣ���ȷ�����Ļ�ѧ��ӦΪ���Ĺؼ���ע��������ԭ���������Ⱥ������йص����ӷ�ӦΪ�����ѵ㣬ѡ��CΪ�״��㣬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�
�����Ŀ
 ��2012?������ģ��A��B��C��D������ѧ��ѧ�г������ʣ�����A��B��C������ͬһ��Ԫ�أ���һ���������ת����ϵ��ͼ�����ַ�Ӧ�е�ˮ����ȥ����
��2012?������ģ��A��B��C��D������ѧ��ѧ�г������ʣ�����A��B��C������ͬһ��Ԫ�أ���һ���������ת����ϵ��ͼ�����ַ�Ӧ�е�ˮ����ȥ����