��Ŀ����
����Ŀ��ʵ����������480mL0.2mol��L-1��NaOH��Һ��
(1)ͨ�������֪��Ӧ��������ƽ����_____��NaOH���塣
(2)��ʵ������Ҫ�IJ�����������Ͳ����ͷ�ιܡ��ձ����_____��
(3)������ѡ������IJ�����д����ȷ��˳��_____������ĸ����
A.ϴ�� B.��ȴ C.��ȡ D.���� E.���� F.ת����Һ G.ϡ�� H.�ܽ�
(4)��ʵ������г������������������Һ��Ũ���к�Ӱ��(����ƫ��������ƫ����������Ӱ����)��
�ٶ���ʱ������ˮ���������˿̶�_____��
������ƿ�������һ����ˮ��_____��
�۶���ʱ���ӹ۲쵽Һ��պõ���̶���_____��
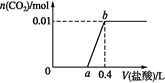
(5)��NaOH��Һ��2.24L����(STP)��ǡ����ȫ��Ӧ��������������Һ_____mL��
(6)��18mol��L-1����������100mL1.0 mol��L-1���ᣬʵ��ʱӦѡ�õ�������_____�����ţ���
A.100mL��Ͳ B.������ƽ C.������ D.50mL����ƿ
E.10mL��Ͳ F.��ͷ�ι� G.50mL�ձ� H.100mL����ƿ
���𰸡�4.0 500mL ����ƿ DHBFAE ƫ�� ��Ӱ�� ƫ�� 1000 CEFGH
��������
(1)����ʵ������ѡ�����ѡ��500mL����ƿ������Һ������c=![]() ��m=nM�������ʵ�������
��m=nM�������ʵ�������
(2)����������Һ�IJ������ʹ�õĸ���������
(3)����������Һ�IJ���������
(4)�������ʵ���Ũ�ȶ���ʽc=![]() ����ʵ����
����ʵ����
(5)����������NaOH��Ӧ�����ʵ�����ϵ���ȼ�����ҪNaOH�����ʵ�����Ȼ�����n=cV���㣻
(6)������������ΪҺ̬���ʼ��������ʵ���Ũ����Һ�IJ�������ȷ��ʹ�õ�������
(1)ѡ��500mL����ƿ������Һ����c=![]() ��֪n=c��V=0.2mol/L��0.5L=0.1mol������Ҫ���������ʵ�����m=nM=0.1mol��40g/mol=4.0g��
��֪n=c��V=0.2mol/L��0.5L=0.1mol������Ҫ���������ʵ�����m=nM=0.1mol��40g/mol=4.0g��
(2)����һ�����һ�����ʵ���Ũ�ȵ���Һ��������Ҫ�IJ�����������Ͳ����ͷ�ιܡ��ձ��Ҫ��һ����������ƿ����ʵ����û��480mL������ƿ������ѡ�������ı��������ԭ��Ҫʹ��500mL������ƿ��
(3)ȷ����һ�����һ�����ʵ���Ũ�ȵ���Һ�IJ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ����Ժ���˳��ΪD��H��B��F��A��E��
A.ϴ�� B.��ȴ C.��ȡ D.���� E.���� F.ת����Һ G.ϡ�� H.�ܽ⣻
(4)�ٶ���ʱ������ˮ���������˿̶ȣ�ʹVƫ�����������ʵ����ʵ������䣬���������Һ��Ũ��ƫ�ͣ�
������ƿ�������һ����ˮ�֣���Ӱ�����ʵ���������Һ���������˶�������Һ��Ũ����Ӱ�죻
�۶���ʱ���ӹ۲쵽Һ��պõ���̶��ߣ���ʹ��Һ�����ƫ�������Ƶ���ҺŨ��ƫ�ͣ�
(5)Cl2��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ��Cl2+2NaOH=NaCl+NaClO+H2O��n(Cl2)=2.24L��22.4L/mol=0.1mol������ݷ���ʽ�����ʷ�Ӧ���Ĺ�ϵ��֪��NaOH�����ʵ���Ϊ0.2mol������n=cV��֪���NaOH��Һ�����V=0.2mol��0.2mol/L=1L=1000mL��
(6) 100mL1.0 mol/L��������ʵ���n(H2SO4)=1.0mol/L��0.1L=0.1mol��������Һ��ϡ��ǰ�����ʵ����ʵ������䣬������18mol/L��Ũ��������V=0.1mol��18mol��L-1=0.0056L=5.6mL��Ũ�����Һ̬������Ҫʹ��10mL����Ͳ��ȡ����ʹ��100mL����Ͳ��������ƽ������ȡ��Ũ����ת����ʢ��һ����ˮ��50mL���ձ��У��ò��������裬ʹ�ܽ�ų�������Ѹ����ɢ����ȴ��ͨ������������ת����100mL������ƿ�У�Ȼ��������ˮϴ���ձ�����������ϴ��ҺҲת��������ƿ�У��ټ�ˮ��Һ����̶���1-2cm�������ý�ͷ�ιܵμ�����Һ����̶������У�ҡ�Ⱦ͵õ�100mL1.0 mol.L���ᣬ��ʹ�õ�������10mL��Ͳ��50mL�ձ�������ƿ��100mL����ƿ����ͷ�ιܣ�����ѡ�������CEFGH��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
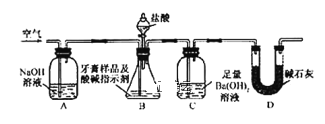
Сѧ��10����Ӧ����ϵ�д�����Ŀ�������̼����(Na2CS3)������ɱ��������������ijС�����ʵ��̽�������̼���Ƶ����ʲ��ⶨ����Һ��Ũ�ȡ�
ʵ��1��̽��Na2CS3������
���� | ���������� |
�� | ȡ����Na2CS3 ������������ˮ���Ƴ���Һ���ֳ����ȷ� |
�� | ������һ����Һ�еμӼ��η�̪��Һ����Һ���ɫ |
�� | ����һ����Һ�еμ�����KMnO4��Һ����ɫ��ȥ |
��1��H2CS3��________�ᣨ����ǿ��������������
��2����֪����۵�����������SO42����д���÷�Ӧ�����ӷ���ʽ________________________��
ʵ��2���ⶨNa2CS3��Һ��Ũ�ȣ�����ͼ��ʾ���Ӻ�װ�ã�ȡ100mLNa2CS3��Һ����������ƿ�У�������d�Ļ�������������2.0mol/LϡH2SO4���رջ�����
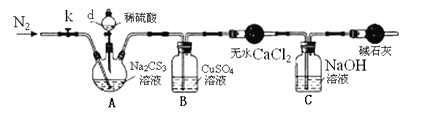
��֪��Na2CS3 + H2SO4=Na2SO4 + CS2 + H2S����CS2��H2S���ж���CS2������ˮ���е�46�棬��CO2ijЩ�������ƣ���NaOH��������Na2COS2��H2O��
��3��ʢ����ˮCaCl2��������������____________��
��4����Ӧ���������k���ٻ���ͨ����N2һ��ʱ�䣬��Ŀ����___________________��
��5��Ϊ�˼���Na2CS3��Һ��Ũ�ȣ���B�л������й��ˡ�ϴ�ӡ�������أ���19.2g���壬��A��Na2CS3�����ʵ���Ũ��Ϊ____________________��
��6����������ʵ�鷽����������ͨ���ⶨC����Һ����������ֵ������Na2CS3��Һ��Ũ�ȣ�����Ӧ������ͨ��N2��Ϊͨ�ȿ���������ֵ________������ƫ��������ƫ����������Ӱ��������