��Ŀ����
�Իش��������⣺��1������ʵ������ѡ�õ�����������Լ����۲���������
A.��������ƽ����11.7g�Ȼ��ƾ���
B.�ü�ʽ�ζ�����ȡ20.00mL Na2CO3��Һ
C.����NH4Cl����ʱ����ʪ���ɫʯ����ֽ�����Թܿڣ�����NH3������
D.�ⶨ��Һ��pHʱ���ýྻ������IJ�����պȡ��Һ������������ˮʪ�����pH��ֽ�������ɫ���Ƚ�
E.��������ˮ��pH��ֽ���Ϳ��Լ���pH��ȵ�H2SO4��CH3COOH��Һ
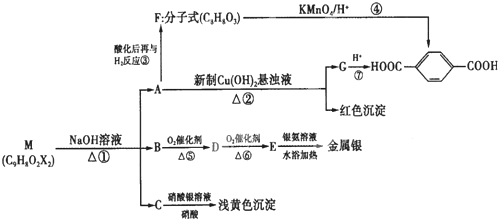
��2������ֲ�ﺣ���к��д����ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڡ�ʵ������Ӻ�������ȡ�����������ͼ��
I.ָ����ȡ��Ĺ������йص�ʵ��������ƣ��� ����
II.��ȡ��Ĺ����У��ɹ�ѡ����л��Լ��� ������ţ�
A.�ױ����ƾ� B.���Ȼ�̼����
C.���͡����� D.���͡�����
III.Ϊʹ��ˮ��Һת��Ϊ����л���Һ��ʵ�������ձ���������������ƿ���ƾ��ơ����ܡ�Բ����ƿ��ʯ�����Լ���Ҫ�ļг���������Ʒ����ȱ�ٵIJ���������
��6�֣���1��CD��2�֣�
��2����4�֣�ÿ��1�֣�I.�ٹ��� ����ȡ II. B III.��Һ©��
����:��




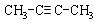
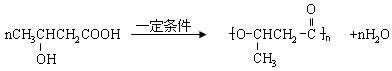
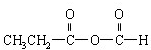
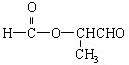
 ��
��


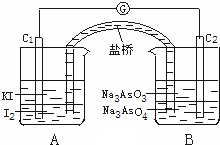 ��֪���淴Ӧ��AsO43-+2I-+2H+?AsO33-+I2+H2O�ݴ���Ƴ���ͼ��ʾ��ʵ��װ�ã�װ�������ŵ�������ʹ����װ���γ�һ���պϻ�·�����������²�����
��֪���淴Ӧ��AsO43-+2I-+2H+?AsO33-+I2+H2O�ݴ���Ƴ���ͼ��ʾ��ʵ��װ�ã�װ�������ŵ�������ʹ����װ���γ�һ���պϻ�·�����������²�����