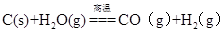��Ŀ����
��1��������һ����Ҫ�Ļ���ԭ�ϡ�Ŀǰ�Ƽҵ��Ҫ�С�������͡������Ƽ���������Ƽ�����ֹ��ա�
�١��������������CaCl2�����д���ù����в���CaCl2�Ļ�ѧ����ʽ��_________________________________________________��
��д���������Ƽ���йط�Ӧ�Ļ�ѧ����ʽ_________________ _�� ��
��CO2���Ƽҵ����Ҫԭ�ϣ��������Ƽ���롰�������CO2����Դ�кβ�ͬ��________________________________________��
��2��������ҵ�Դٽ����ú���ᷢչ������Ҫ���á�
������ʱ������衢�̺�����Ŀ����_______________________________��
�ڲ���ֺ��е�CrԪ���������ֹ��̵�����__ __���ǰ�������롣
�����������������У�β�������е���Ҫ��Ⱦ����________���ӻ����;��ýǶȿ��ǣ�����β��������������_________��
�١��������������CaCl2�����д���ù����в���CaCl2�Ļ�ѧ����ʽ��_________________________________________________��
��д���������Ƽ���йط�Ӧ�Ļ�ѧ����ʽ_________________ _�� ��
��CO2���Ƽҵ����Ҫԭ�ϣ��������Ƽ���롰�������CO2����Դ�кβ�ͬ��________________________________________��
��2��������ҵ�Դٽ����ú���ᷢչ������Ҫ���á�
������ʱ������衢�̺�����Ŀ����_______________________________��
�ڲ���ֺ��е�CrԪ���������ֹ��̵�����__ __���ǰ�������롣
�����������������У�β�������е���Ҫ��Ⱦ����________���ӻ����;��ýǶȿ��ǣ�����β��������������_________��
��1����2NH4Cl��Ca(OH)2 2NH3����CaCl2��2H2O ��2�֣�
2NH3����CaCl2��2H2O ��2�֣�
��NH3��CO2��H2O��NaCl�����ͣ���NaHCO3����NH4Cl ��2�֣�
2NaHCO3 Na2CO3��CO2����H2O ��2�֣�
Na2CO3��CO2����H2O ��2�֣�
����д�ܷ�Ӧ����ʽ��2NaCl��2NH3��CO2��H2O��Na2CO3��2NH4Cl��
�ۡ������CO2��Դ��ʯ��ʯ���գ��������Ƽ��CO2��Դ�ںϳɰ���ҵ�ķ�������2�֣�
��2���������͵����ֵijɷ� ��2�֣�
�ں� ��1�֣�
��CO ��2�֣� ȼ�ϣ���ԭ���� ��2�֣�
 2NH3����CaCl2��2H2O ��2�֣�
2NH3����CaCl2��2H2O ��2�֣���NH3��CO2��H2O��NaCl�����ͣ���NaHCO3����NH4Cl ��2�֣�
2NaHCO3
 Na2CO3��CO2����H2O ��2�֣�
Na2CO3��CO2����H2O ��2�֣�����д�ܷ�Ӧ����ʽ��2NaCl��2NH3��CO2��H2O��Na2CO3��2NH4Cl��
�ۡ������CO2��Դ��ʯ��ʯ���գ��������Ƽ��CO2��Դ�ںϳɰ���ҵ�ķ�������2�֣�
��2���������͵����ֵijɷ� ��2�֣�
�ں� ��1�֣�
��CO ��2�֣� ȼ�ϣ���ԭ���� ��2�֣�
�����������1�� �ٰ����Ϊ���հ���ʹʯ�����븱�����Ȼ�立�Ӧ���Ӷ���������CaCl2������2NH4Cl + Ca(OH)2=CaCl2+2H2O+2NH3�����������Ƽ����Ҫ��ѧ��ӦΪ��
NaCl(����)+CO2+NH3+H2O=NaHCO3��+NH4Cl 2NaHCO3
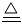 Na2CO3+H2O+CO2��
Na2CO3+H2O+CO2���۰����CO2��Դ��ʯ��ʯ�����գ��������Ƽ����CO2��Դ�ںϳɰ���ҵ�ķ�����
��2��������ʱ������衢�̺�����Ҫ�ǿ��������͵����ֵijɷ֣��� ��ΪCr�ױ�������Ϊ��ֹCr������������ֺ��е�CrԪ���������ֹ��̵�������������ǰ����Cr���γ�¯������ȥ�������������������У�CO����Ҫ�Ļ�ԭ������β�������е���Ҫ��Ⱦ����CO��һ����̼�������ж������������д�����

��ϰ��ϵ�д�
�����Ŀ

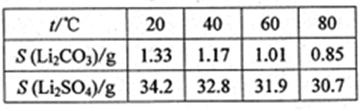
 VOA2(�л��㣩+ H2SO4(ˮ��)��������п�ѡ������������ȡ��ԭ����_____________��
VOA2(�л��㣩+ H2SO4(ˮ��)��������п�ѡ������������ȡ��ԭ����_____________��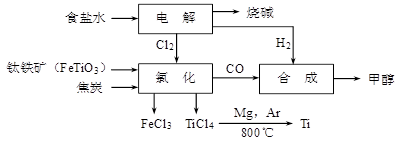
 2Hg��O2��
2Hg��O2�� 2Al2O3��3Mn
2Al2O3��3Mn 2Al��3Cl2 ��
2Al��3Cl2 ��
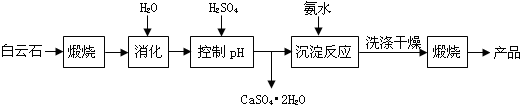
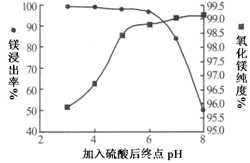
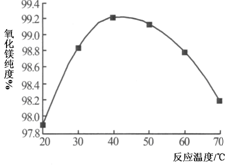
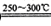 Li2SO4+Al2O3·4SiO2?H2O
Li2SO4+Al2O3·4SiO2?H2O