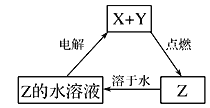
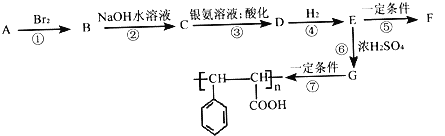
��Ŀ����
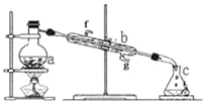
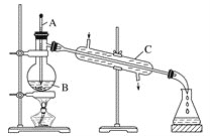
����Ŀ�����;�ˮ���������(K2FeO4)Ϊ����ɫ���壬������ˮ�������Ի�������Һ���ֽ⣬�ڼ�����Һ���ȶ�����ҵ���Ʊ�K2FeO4�ij��÷��������֡�
������������������������������ͼ��ʾ��
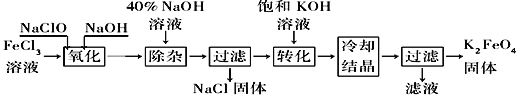
��1����ɡ������������з�Ӧ�Ļ�ѧ����ʽ��______FeCl3��______NaOH��______NaClO==______Na2FeO4��______![]() ��______
��______![]() ��____________������������________(�ѧʽ)��
��____________������������________(�ѧʽ)��
��2����ת���������з�����Ӧ�Ļ�ѧ����ʽΪ____________________________________��
��3���������յõ��ĸ�����س��������ʣ������ؽᾧ���ᴿ�������ǽ��ֲ�Ʒ��________________�ܽ⣬Ȼ��________________��
������ⷨ������Ϊ�����������������Һ��Ȼ��������Һ�м���KOH��
��4�����ʱ����������Ӧ����FeO42�����õ缫��Ӧ����ʽΪ________________________________��
���𰸡�2��10��3��2��9��NaCl��5��H2ONaClONa2FeO4��2KOH===K2FeO4��2NaOHϡKOH��Һ���뱥��KOH��Һ����ȴ�ᾧFe��8OH����6e��=FeO42-��4H2O
��������
��1��Fe�Ļ��ϼ��ɣ�3�ۡ���6�ۣ����ϼ�����3�ۣ�Cl�Ļ��ϼ��ɣ�1�ۡ���1�ۣ����ϼ۽���2�ۣ���С������Ϊ6��Ȼ�����ԭ���غ���ƽ�������ɣ�����Ӧ����ʽΪ2FeCl3��10NaCl��3NaClO=2Na2FeO4��9NaCl��5H2O������NaClOΪ����������2���������̣����˺���Һ�к���Na2FeO4������K2FeO4���ܽ��С��Na2FeO4����˷����ķ�Ӧ����ʽΪNa2FeO4��2KOH=K2FeO4��2NaOH����3����Ϊ������������Ի�������Һ���ֽ⣬�ڼ�����Һ���ȶ����ڣ��������ϡKOH��Һ�ܽ⣬Ȼ����뱥��KOH��Һ����ȴ�ᾧ����4�������ΪKOH��������������������缫��ӦʽΪFe��8OH����6e��=FeO42����4H2O��

����Ŀ���±���Ԫ�����ڱ���һ���֣���Ա��еĢ١�����Ԫ�أ���Ԫ�ط��Ż�ѧʽ��ջش��������⣺
IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 | |
�� | �� | �� | ||||||
�� | �� | �� | �� | �� | �� | �� | ||
�� | �� | �� |
(1)����ЩԪ���У�����ʧ���ӵ�Ԫ����________���ǽ�������ǿ��Ԫ����______��
(2)��ѧ��������õ�Ԫ����_____����ԭ�ӵ�ԭ�ӽṹʾ��ͼΪ________��
(3)Ԫ�ص�����������Ӧ��ˮ������������ǿ����____��������ǿ����___�������Ե�����������_______________��(��д��ѧʽ)
(4)�ڢۡ���Ԫ���У������Ӱ뾶��С����_________��
(5�ڢ����ĵ����У������Խ�ǿ����_______________���÷�Ӧ��ѧ����ʽ֤����_____________________________��