��Ŀ����
��14�֣�
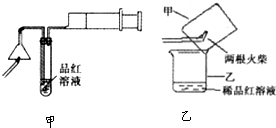
ij�о���ѧϰС��Ϊ�о�Cu��ŨH2SO4�ķ�Ӧ���������ʵ��̽��������װ���еĹ̶������;ƾ��ƾ�δ��������
ʵ��ѡ��ͭƬ��98.3%��H2SO4��Ʒ����Һ������ʯ��ˮ��CCl4��NaOH��Һ��ҩƷ��ͭƬһ��û��ŨH2SO4�У���һ��¶����Һ���Ϸ���
�ش��������⡣
��1��Cu��ŨH2SO4�ķ�Ӧ�Ļ�ѧ����ʽΪ____________________��
��2��D��E��������CCl4��������____________________��
��3�����ȹ����У��۲쵽A�����г��ִ�����ɫ���������ŷ�Ӧ�Ľ��У�A�������а�ɫ�������ɣ�����Ϊ�ó�������_________________���������ܵ�ԭ����____________________��
��4����A�����е�ŨH2SO4��ͭƬ���м��ȣ��ܿ췢��C������Ʒ����Һ��ɫ����ʼ��δ��D�Թ��г���ʯ��ˮ���ֻ��ǻ��������IJ�����__________________�����ʵ����֤��IJ���__________________��
��5��ʵ�������Ϊ�˼��ٻ�����Ⱦ���ų���װ���е�SO2���ɲ�ȡ�IJ�����___________��
��1�� Cu+2H2SO4��Ũ����CuSO4+SO2��+H2O��2�֣� ��2�� ��ֹ������2�֣�
��3��CuSO4 ��2�֣��� Ũ�����к�ˮ�٣����ɵ�����ͭ�϶࣬Ũ�������ˮ���ã�2�֣�
��4������SO2�ܽ�Ƚϴ���ʯ��ˮ��Ca��OH��2�����ͣ�������Ca��HSO3��2��Һ��Ե�ʣ�2�֣���ȡ���������м�������������Һ���۲��Ƿ��г������ɣ�2�֣��������ȡ����������SO2����ȷ�����
��5����A�����ϵĵ��ɼУ�����ͨ���������װ���е�SO2�ϵ�E�У��������B�м���NaOH��Һ�������ӣ����ɣ�2�֣���ע��ֱ����A�м�NaOH��Һ�����֣�
����:

 ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�| ���� | 0�� | 10�� | 20�� | 30�� | 40�� | 50�� | 60�� | 100�� |
| NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 39.8 |
| NH4HCO3 | 11.9 | 15.8 | 21.0 | 27.0 | 35�����Ϸֽ� | |||
| NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 | 16.4 | - |
| NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.3 | 77.3 |
| A����һ��������Ӧ�Ļ�������Ϊ���Ϸ�Ӧ���ֽⷴӦ |
| B����һ��������Ӧ֮�����Ҫʵ������ǹ��ˡ�ϴ�� |
| C����һ����Ӧ�����¶ȸ���30��Ŀ������߷�Ӧ���� |
| D����ӦҺ�����ᴦ����ʹNaClѭ��ʹ�ò�����NH4Cl |
 ��һ�ֺ�������Ҫ�ɷ���Fe2O3���������ʲ�����ˮ���ᣮij�о���ѧϰС���ͬѧ������һС����Ʒ������������ʵ�飮
��һ�ֺ�������Ҫ�ɷ���Fe2O3���������ʲ�����ˮ���ᣮij�о���ѧϰС���ͬѧ������һС����Ʒ������������ʵ�飮