��Ŀ����
�����Ȼ�ѧ����S ( s ) + O2 ( g ) = SO2 ( g ) ��H = ��297.23 kJ/mol ����Ӧ��25�棬101kPa�·�������������˵����ȷ����
| A�������Ȼ�ѧ����ʽ��ȼ��1mol S�ų�������Ϊ297.23 kJ |
| B���γ�1 mol SO2�Ļ�ѧ�����ͷŵ����������ڶ��� 1 mol S ( s )�� 1mol O2 ( g )�Ļ�ѧ�������յ������� |
| C��S ( g ) + O2 ( g ) = SO2 ( g )��H =��Q kJ/mol��Q>297.23 |
| D��S ( g ) + O2 ( g ) = SO2 ( g )��H =��Q kJ/mol��Q<297.23 |
BC
A������ȷ����Ӧ��25�棬101kPa�·�����ȼ��1mol S��������SO2���壬�ų�������Ϊ297.23 kJ��B����ȷ������S ( s ) + O2 ( g ) = SO2 ( g )�Ƿ��ȷ�Ӧ��C����������ʱҪ���ȣ���C��ȷ��D����ȷ��ѡBC��

��ϰ��ϵ�д�
 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д�
�����Ŀ
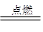 2H2O���Իش��������⡣
2H2O���Իش��������⡣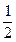 O2��g��====H2O��l����H����285kJ��mol��1
O2��g��====H2O��l����H����285kJ��mol��1 ����5O2��g��====3CO2��g����4H2O��l����H����2220.0kJ��mol��1
����5O2��g��====3CO2��g����4H2O��l����H����2220.0kJ��mol��1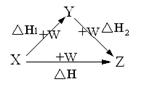
 O2(g)��H2O(l) ����H 3����285.8kJ/mol
O2(g)��H2O(l) ����H 3����285.8kJ/mol  �ܱ������У�����1 mol CO2��3 mol H2��һ�������·�����Ӧ��CO2(g)��3H2(g)
�ܱ������У�����1 mol CO2��3 mol H2��һ�������·�����Ӧ��CO2(g)��3H2(g) CH3OH(g)��H2O(g) ��H����49.0kJ/mol
CH3OH(g)��H2O(g) ��H����49.0kJ/mol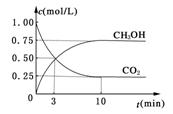
 ��H����1277 kJ��mol-1
��H����1277 kJ��mol-1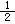 O2(g)��CO(g)�� ��H����110.5 kJ��mol-1
O2(g)��CO(g)�� ��H����110.5 kJ��mol-1 ���й���ѧԺ����Ӧ�û�ѧ�о����ڼ״�ȼ�ϵ�ؼ�����������ͻ�ƣ���װ�����Ժ�����ؼ�����ʽ��ѡ��״�ȼ�ϵ�صĹ���ԭ������ͼ��ʾ��
���й���ѧԺ����Ӧ�û�ѧ�о����ڼ״�ȼ�ϵ�ؼ�����������ͻ�ƣ���װ�����Ժ�����ؼ�����ʽ��ѡ��״�ȼ�ϵ�صĹ���ԭ������ͼ��ʾ��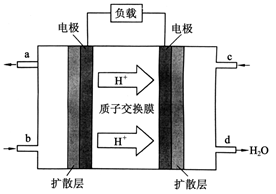

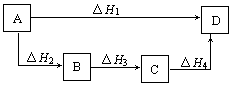
 4
4