��Ŀ����
����Ŀ��2Zn(OH)2ZnCO3���Ʊ�����ZnO���м��壬��п��ɰ����Ҫ�ɷ�ΪZnO��������Cu2+��Mn2+�����ӣ�Ϊԭ���Ʊ�2Zn(OH)2ZnCO3�Ĺ����������£�

��ش��������⣺
��1����(NH4)2SO4��NH3��H2O�Ļ����Һ�д���c(NH4+)��2c(SO42 - )ʱ����Һ�� �����������������������������ԡ�
��2������ȡ��ʱΪ�����п�Ľ����ʣ��ɲ�ȡ�Ĵ�ʩ�� ����дһ�֣���
��3������ȡ��ʱ�����NH3��H2O����������MnO2�����ӷ���ʽΪ ��
��4������S2-�ܽ�Cu2+������ת��Ϊ�����������ȥ����ѡ��ZnS���г��ӣ��Ƿ���У��ü���˵��ԭ�� ��
[��֪��Ksp(ZnS)��1.6��10-24��Ksp(CuS)��1.3��10-36]
��5������п�������ӷ���ʽΪ ��
��6��������3��������Һ��ѭ��ʹ�ã�����Ҫ�ɷֵĻ�ѧʽ�� ��
��������1���У���2�����衢�ʵ����ȣ�3��Mn2+��H2O2��2NH3��H2O��MnO2����2NH4+��2H2O
��4��������ZnS��Cu2+��CuS��Zn2+��K��![]() ��1.2��1012����1��105
��1.2��1012����1��105
��5��3Zn2+��6HCO3����2Zn��OH��2ZnCO3����5CO2����H2O��6����NH4��2SO4
��������
�����������1����������غ��֪c��H+��+c��NH4����= c��OH������2c��SO42��������Һ��c��NH4������2c��SO42����������c��H+��= c��OH�����������Һ�����ԡ�
��2������ȡ��ʱΪ�����п�Ľ����ʣ��ɲ�ȡ�Ĵ�ʩ�����衢�ʵ�������
��3������ȡ��ʱ�����NH3��H2O��������������Һ��˫��ˮ��������������MnO2�����ӷ���ʽΪMn2+��H2O2��2NH3��H2O��MnO2����2NH4+��2H2O��
��4����������ʽZnS��Cu2��CuS��Zn2+��֪����Ӧ��ƽ�ⳣ��K��![]() =
=![]() ��1.2��1012����1��105������ǿ��еġ�
��1.2��1012����1��105������ǿ��еġ�
��5������ԭ���غ��֪����п��ʱ���ɵ�A�����Ƕ�����̼����Ӧ�����ӷ���ʽΪ3Zn2+��6HCO3 -��2Zn��OH��2ZnCO3����5CO2����H2O��
��6��������3��������Һ����������泥���ѭ��ʹ�ã�����淋Ļ�ѧʽΪ��NH4��2SO4��

 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д� ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�����Ŀ�����÷�ӦZn��2FeCl3===ZnCl2��2FeCl2���һ��ԭ��ء�
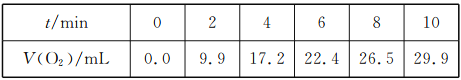
��1������ͼ�����ڻ���ʵ��װ��ͼ��
��2��ָ����������Ϊ__________���缫��ӦʽΪ______________________��
��������Ϊ____________���缫��Ӧ����Ϊ_________________________��
��3�����·�еĵ����Ǵ�____________������__________����