��Ŀ����
18������ɲ�ͬ��3�ֺ��������ֱ��� H2[PtCl4��OH��2]����NH4��2[PtCl6]��H2[PtCl2��OH��4]����Һ��������֮�������µ�ת����ϵ��2H2[PtCl4��OH��2]+2NH3=��NH4��2[PtCl6]+H2[PtCl2��OH��4]��������3�ֺ����������˵����ȷ���ǣ�������| A�� | H2[PtCl2��OH��4]���к�ǿ�ļ��� | B�� | 3�ֺ������������λ����Ϊ6 | ||
| C�� | 3�ֺ��������ﶼ���ڹ��ۻ����� | D�� | 3�ֺ����������Pt�Ļ��ϼ۲�ͬ |
���� �����Ҳ������Ϊһ�����������ѧ�ṹ�Ļ����������ԭ�ӻ����ӣ�ͳ������ԭ�ӣ���Χ�����ij�Ϊ��λ�壨������壩�ķ��ӻ����ӣ���ȫ������λ������γɣ������������ԭ���ṩ�չ���������ṩ�µ��Ӷԣ�
A���������м��ԣ����ݰ�����H2[PtCl2��OH��4]����ʽ������
B�������H2[PtCl4��OH��2]�У�Pt4+Ϊ�������ӣ�Cl-��OH-Ϊ���壬��NH4��2[PtCl6]�У�Pt4+Ϊ�������ӣ�Cl-Ϊ���壬H2[PtCl2��OH��4]�У�PtΪ�������ӣ�Cl-��OH-Ϊ���壬��������ĸ����ж���λ����
C����NH4��2[PtCl6]�������ӻ����
D��3�ֺ����������Pt�Ļ��ϼ۶�Ϊ+4�ۣ�
��� �⣺A������Ϊ���м��Ե����壬�����������H2[PtCl2��OH��4]��Ӧ��2H2[PtCl4��OH��2]+2NH3=��NH4��2[PtCl6]+H2[PtCl2��OH��4]��Ϊ������������������ӷ�Ӧ������H2[PtCl2��OH��4]�����к�ǿ�ļ��ԣ���A����
B�������H2[PtCl4��OH��2]�У�Pt4+Ϊ�������ӣ�Cl-��OH-Ϊ���壬��λ��Ϊ6����NH4��2[PtCl6]�У�Pt4+Ϊ�������ӣ�Cl-Ϊ���壬��λ��Ϊ6��H2[PtCl2��OH��4]�У�Pt4+Ϊ�������ӣ�Cl-��OH-Ϊ���壬��λ��Ϊ6����B��ȷ��
C��H2[PtCl4��OH��2]��H2[PtCl2��OH��4]Ϊ���ۻ������NH4��2[PtCl6]Ϊ笠�����Ϊ������ӵ��Σ��������ӻ������C����
D�������H2[PtCl4��OH��2]�У�Pt4+Ϊ�������ӣ���NH4��2[PtCl6]�У�Pt4+Ϊ�������ӣ�H2[PtCl2��OH��4]�У�Pt4+Ϊ�������ӣ�Pt�Ļ��ϼ۶�Ϊ+4�ۣ���D����
��ѡB��
���� ���⿼�������ijɼ������ע�����塢�������ӡ���������Լ���λ�����жϣ�������ظ���ر�ע�������������ӵ�������Ŀ�Ѷ��еȣ�

| A�� | ���ά��������ά���������ڸ߷��ӻ����� | |
| B�� | �ڴ�����ƵĹ����У�����������˵��� | |
| C�� | ������Ư�۴�������ˮ�����ߵ�����ԭ����ͬ | |
| D�� | �ϳ�˳���� ���ĵ�����CH2=CH-CH=CH2�� ���ĵ�����CH2=CH-CH=CH2�� |
| A�� | ��ϵͳ������������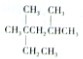 ��������2��4-����-2-�һ����� ��������2��4-����-2-�һ����� | |
| B�� | ��ϩ�ᣨCH2=CH-COOH���ܷ���ȡ���Ӿ����۷�Ӧ | |
| C�� | ���ࡢ��֬�������ʶ��Ǹ߷��ӻ����һ�������¶��ܷ���ˮ�ⷴӦ | |
| D�� | �����ʵ�����HCOOCH3��CH3CHO��ȫȼ�գ������ͨ�������Ĺ������Ʋ���ַ�Ӧ������������ͬ |
| A�� | ͨ������CO2�����Һ�У�Na+��SO32-��CH3COO-��HCO3- | |
| B�� | ��ɫ��Һ�У�Mg2+��MnO4-��SO42-��K+ | |
| C�� | $\frac{c��{H}^{+}��}{c��O{H}^{-}��}$=1012����Һ�У�NH4+��Fe2+��NO3-��Cl- | |
| D�� | c��ClO-��=1.0 mol•L-1����Һ��Na+��SO32-��S2-��SO42- |
| A�� | ��ˮ�С�Ԫ�ع��硱֮�ƣ��屻��Ϊ������Ԫ�ء� | |
| B�� | ��չ�ȼҵ�����ú�ˮ��һ���������������������H2�ǻ�ԭ���� | |
| C�� | ��ҵ�ϴӺ�ˮ�л�ȡNaCl��AlCl3��ͨ������������οɻ�ý���Na��Al | |
| D�� | ���ú�ˮͨ�������仯���Ի�õ�ˮ��ʳ�Σ����Ӻ�ˮ�л�ȡMgCl2��Br2����ͨ����ѧ�仯 |
| A�� | c��H+��=1��10-14mol/L����ɫ��Һ��K+��S2-��MnO4-��SO42- | |
| B�� | ˮ�������c��H+��=1��10-14mol/L����Һ��K+��Na+��AlO2-��S2O32- | |
| C�� | ����Al��Ӧ����H2����Һ��NH4+��Ca2+��NO3-��I- | |
| D�� | ��ʹ���ȱ�����Һ�У�Na+��NH4+��Fe2+��NO3- |
| A�� | ������������Դ�Ϳ�������Դ | |
| B�� | ���д�����չ�����ͨ���ٿ�˽�ҳ� | |
| C�� | ���ȼú�����ȼ������ | |
| D�� | ѧУ����ͥ��װ��������װ�� |

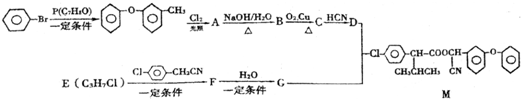
 ��
��