��Ŀ����
����aA��bB��cC��dD��eE���ֶ�����Ԫ�أ����Ƕ��������в���ȱ�ٵ���ҪԪ�ء���֪���ǵ�ԭ�����������¹�ϵ��a��b��c��a��c��d��c��d��e��B��D��E���ж���ͬ�������壬B�Ļ������������A�Ļ�����������������������顣�ݴˣ��ش������й����⣺(1)д������Ԫ�ص�Ԫ�ط��ţ�B________��D________��д��E�����ֳ���ͬ�������������________��C��E���⻯��ķе�ߵ�ϵ��________(�÷���ʽ�͡�������ʾ)��
(2)X��C������������ˮ���������Ҫ�Ļ�ѧ�Լ�����Ũ��Һ��ܹⱣ�棬������Ϊ(�û�ѧ����ʽ��ʾ)____________________________________��
(3)Y��C������������ˮ��������⻯�ﷴӦ���ɵĻ��������ʱ��pH=a��X��Y������Һ����ˮ���������H+Ũ��֮��Ϊ__________________��
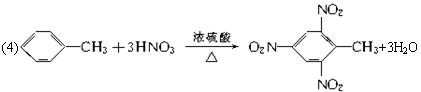
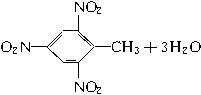
(4)X��A��B��ɵ�ij��������һ�������·�Ӧ�Ƶ�һ�ֳ���������ըҩ���䷴Ӧ�Ļ�ѧ����ʽΪ____________________________________��
(5)������Ԫ����ɵ�ij�������仯ѧʽΪB
(1)C O ���ס����� NH3��PH3
(2)4HNO3![]() 4NO2��+O2��+2H2O
4NO2��+O2��+2H2O
(3)
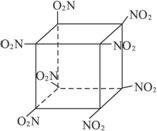
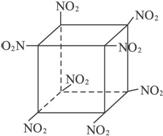
(5)
��������ԭ�������Ĺ�ϵ��a+b=c��a+c=d��c+d=e��B��D��E���ж���ͬ����������֪a=1��b=6��c=7��d=8��e=15������A��H��B��C��C��N��D��O��E��P��
(1)NH3���������з��Ӽ���������Ա�ͬ�������̬�⻯��е�ߡ�
(3)HNO3��Һ������ˮ�ĵ��룬NH4NO3����������Ϊ![]() ��ˮ�⣬�ٽ���ˮ�ĵ��룬pH=a��HNO3��NH4NO3��ˮ���������H+Ũ��֮��Ϊ10-14+a��10-a=
��ˮ�⣬�ٽ���ˮ�ĵ��룬pH=a��HNO3��NH4NO3��ˮ���������H+Ũ��֮��Ϊ10-14+a��10-a=
(4)����ըҩΪ�������ױ����ʷ�ӦΪ��
![]()
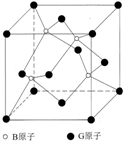
(5)������C8N8O16���������Ϣ�ó��ṹΪ��




 ����aA��bB��cC��dD��eE��gG���ֶ�����Ԫ�أ�a+b=c��a+c=d��a+d=e��d+e=g��B��C��E��G�ĵ��ʾ��ж���ͬ�������壬��ش��������⣺
����aA��bB��cC��dD��eE��gG���ֶ�����Ԫ�أ�a+b=c��a+c=d��a+d=e��d+e=g��B��C��E��G�ĵ��ʾ��ж���ͬ�������壬��ش��������⣺