��Ŀ����
��2009?���ڶ�ģ���ȼ���������ࣩ�к���þ�������Ĺ����κ�̼���Σ����к�þ����MgO�ƣ�Լ10%���ƣ���CaO�ƣ�Լ15%�����������ȵĺ�������1%���ȼ����������ȡMgSO4��7H2O���������£�
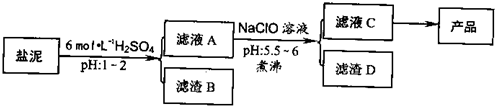
�����������������������ʱ��ҺpH
�ܽ�ȱ�
�ش��������⣺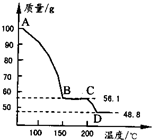
��1������B����Ҫ�ɷ��ǣ�
��2������ҺA�õ���ҺC���ܷ��ð�ˮ����NaClO��
��3������ҺC�л�ò�Ʒ����3���������裬�ֱ���
��4����һ��������MgSO4��7H2O���������м��Ȳ�ò�ͬ�¶Ƚ�ʣ�����������ͼ��ʾ��
��ͼд��CD�η�Ӧ�Ļ�ѧ����ʽ

�����������������������ʱ��ҺpH
| ������ | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Mg��OH��2 |
| pH | 5.2 | 3.2 | 9.7 | 11.2 |
| �¶�0C | 10 | 30 | 40 |
| CaSO4 | 0.19 | 0.21 | 0.21 |

��1������B����Ҫ�ɷ��ǣ�
H2SiO3����H4SO4��\CaSO4����CaSO4?2H2O��
H2SiO3����H4SO4��\CaSO4����CaSO4?2H2O��
����2������ҺA�õ���ҺC���ܷ��ð�ˮ����NaClO��
��
��
��ʲô������ˮ���ܽ�Fe2+������Fe3+������������ȫ
��ˮ���ܽ�Fe2+������Fe3+������������ȫ
�����м�����е�Ŀ����������ʹFe3+��Al3+ˮ����ȫ��ͬʱ��Ϊ�������������������ǽ�״�����������������ƻ�������ȶ��ԣ�ʹ��������������ں�����˷���
������ʹFe3+��Al3+ˮ����ȫ��ͬʱ��Ϊ�������������������ǽ�״�����������������ƻ�������ȶ��ԣ�ʹ��������������ں�����˷���
����3������ҺC�л�ò�Ʒ����3���������裬�ֱ���
����Ũ��
����Ũ��
����ȴ�ᾧ
��ȴ�ᾧ
������
����
����4����һ��������MgSO4��7H2O���������м��Ȳ�ò�ͬ�¶Ƚ�ʣ�����������ͼ��ʾ��
��ͼд��CD�η�Ӧ�Ļ�ѧ����ʽ
MgSO4?H2O
MgSO4+H2O��
| ||
MgSO4?H2O
MgSO4+H2O��
��
| ||
��������1���ں���þ�������Ĺ����κ�̼�����м�����������ɹ��������Ƴ�����
��2������ҺA�õ���ҺC��Ŀ���ǽ�Fe2+������Fe3+��NaClO�������������ã������ˮ��Ϊ���ȹ��̣�����ٽ�ˮ�����ɳ�������ֹ���ɽ��壻
��3������Һ�л�ȡ���壬Ӧ��������Ũ������ȴ�ᾧ�����˵Ȳ�����
��4���������ʵ������仯�ж����ʵ���ɣ����Դ��жϿ��ܷ����ķ�Ӧ��
��2������ҺA�õ���ҺC��Ŀ���ǽ�Fe2+������Fe3+��NaClO�������������ã������ˮ��Ϊ���ȹ��̣�����ٽ�ˮ�����ɳ�������ֹ���ɽ��壻
��3������Һ�л�ȡ���壬Ӧ��������Ũ������ȴ�ᾧ�����˵Ȳ�����
��4���������ʵ������仯�ж����ʵ���ɣ����Դ��жϿ��ܷ����ķ�Ӧ��
����⣺��1���ں���þ�������Ĺ����κ�̼�����м�����������ɹ��������Ƴ�����ΪH2SiO3����H4SO4��\CaSO4����CaSO4?2H2O����
�ʴ�Ϊ��H2SiO3����H4SO4��\CaSO4����CaSO4?2H2O����
��2������ҺA�õ���ҺC��Ŀ���ǽ�Fe2+������Fe3+������ˮ�����ɳ���������ˮ���ܽ�Fe2+������Fe3+������������ȫ��NaClO�������������ã������ˮ��Ϊ���ȹ��̣�����ٽ�ˮ�����ɳ�������ֹ���ɽ��壬������������ƻ�������ȶ��ԣ�ʹ��������������ں�����˷��룬
�ʴ�Ϊ����ˮ���ܽ�Fe2+������Fe3+������������ȫ�� ������ʹFe3+��Al3+ˮ����ȫ��ͬʱ��Ϊ�������������������ǽ�״�����������������ƻ�������ȶ��ԣ�ʹ��������������ں�����˷��룻
��3������Һ�л�ȡ���壬Ӧ��������Ũ������ȴ�ᾧ�����˵Ȳ������ʴ�Ϊ������Ũ������ȴ�ᾧ�����ˣ� ��4��100gMgSO4��7H2O�У�m��MgSO4��=100��
=48.8g��m��H2O��=100g-48.8g=51.2g��
��C������к���ˮ������Ϊ56.1g-48.8g=7.3g����ʱn��H2O��=
=0.41mol��n��MgSO4��=
=0.41mol��
����C��ʹ����Ļ�ѧʽΪMgSO4?H2O��D�����ΪMgSO4����CD�η�Ӧ�Ļ�ѧ����ʽΪMgSO4?H2O
MgSO4+H2O����
�ʴ�Ϊ��MgSO4?H2O
MgSO4+H2O����
�ʴ�Ϊ��H2SiO3����H4SO4��\CaSO4����CaSO4?2H2O����
��2������ҺA�õ���ҺC��Ŀ���ǽ�Fe2+������Fe3+������ˮ�����ɳ���������ˮ���ܽ�Fe2+������Fe3+������������ȫ��NaClO�������������ã������ˮ��Ϊ���ȹ��̣�����ٽ�ˮ�����ɳ�������ֹ���ɽ��壬������������ƻ�������ȶ��ԣ�ʹ��������������ں�����˷��룬
�ʴ�Ϊ����ˮ���ܽ�Fe2+������Fe3+������������ȫ�� ������ʹFe3+��Al3+ˮ����ȫ��ͬʱ��Ϊ�������������������ǽ�״�����������������ƻ�������ȶ��ԣ�ʹ��������������ں�����˷��룻
��3������Һ�л�ȡ���壬Ӧ��������Ũ������ȴ�ᾧ�����˵Ȳ������ʴ�Ϊ������Ũ������ȴ�ᾧ�����ˣ� ��4��100gMgSO4��7H2O�У�m��MgSO4��=100��
| 120 |
| 246 |
��C������к���ˮ������Ϊ56.1g-48.8g=7.3g����ʱn��H2O��=
| 7.3g |
| 18g/mol |
| 48.8g |
| 120g/mol |
����C��ʹ����Ļ�ѧʽΪMgSO4?H2O��D�����ΪMgSO4����CD�η�Ӧ�Ļ�ѧ����ʽΪMgSO4?H2O
| ||
�ʴ�Ϊ��MgSO4?H2O
| ||
���������⿼�����ʵ��Ʊ������롢�ᴿ��������ƣ���Ŀ�Ѷ��еȣ��״���Ϊ��4����ע����������ı仯�������ʵ���ɣ�������д���ܷ�Ӧ�Ļ�ѧ����ʽ��

��ϰ��ϵ�д�
 �߽�������ϵ�д�
�߽�������ϵ�д�
�����Ŀ