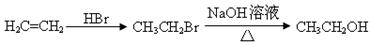��Ŀ����
A�ķ���ʽΪC2H6O2����AΪԭ�ϣ�����ͼ��ʾ���̺ϳ�һ�ֳ��õĹ����߷��Ӳ���G����ṹ��ʽΪ��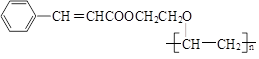 ��
��
��֪���١�CH2OH+��CH2OH ��CH2OCH2��+H2O
��CH2OCH2��+H2O
��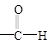 +
+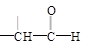
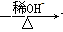
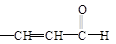 +H2O
+H2O
��ش��������⣺
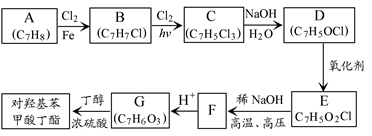
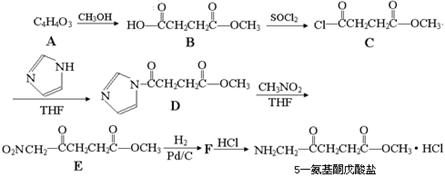
��1��A�������� ��F����G�ķ�Ӧ������
B�ĺ˴Ź��������Ϲ��� �����շ塣
C�Ľṹ��ʽ�� ��
A����B�Ļ�ѧ����ʽ�� ��
B+D����E�Ļ�ѧ����ʽ�� ��
д��ͬʱ��������������D������ͬ���칹��Ľṹ��ʽ��
�����������ұ�����ֻ��һ��ȡ���� �ڳ������ⲻ�ٺ�������״�ṹ
��1���Ҷ��� �Ӿ۷�Ӧ����2��3��
��3��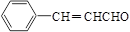
��4��2HOCH2CH2OH HOCH2CH2OCH2CH2OH+H2O
HOCH2CH2OCH2CH2OH+H2O
��5�� +HO(CH2)2O(CH2)2OH
+HO(CH2)2O(CH2)2OH
 CH=CHCOO(CH2)2O(CH2)2OH+ H2O
CH=CHCOO(CH2)2O(CH2)2OH+ H2O
��6��(û��) ��
�� ��
��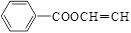 ��
��
���������������G�����֪F����G�ǼӾ۷�Ӧ��F�� CH=CHCOO(CH2)2OCH=CH2���ɷ�Ӧ������֪E��
CH=CHCOO(CH2)2OCH=CH2���ɷ�Ӧ������֪E�� CH=CHCOO(CH2)2O(CH2)2OH��B��HOCH2CH2OCH2CH2OH����ͬ��HΪ3��������Ϣ�ٵó�A���Ҷ�����DΪ
CH=CHCOO(CH2)2O(CH2)2OH��B��HOCH2CH2OCH2CH2OH����ͬ��HΪ3��������Ϣ�ٵó�A���Ҷ�����DΪ ���ɷ�Ӧ������������֪C��
���ɷ�Ӧ������������֪C��
��6����Ϊ֧��ֻ��һ��Oԭ�ӣ�û�������ͬ���칹�塣
���㣺�л���ѧ�ƶϣ������л�������ơ���Ӧ���ͼ�����ʽ��ͬ���칹��Ľṹ��ʽ��

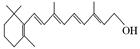
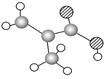
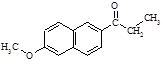 ���⣬��������ɵĸ������G��Ϊͬ���칹�壩�Ľṹ��ʽΪ ��
���⣬��������ɵĸ������G��Ϊͬ���칹�壩�Ľṹ��ʽΪ �� ����������X��C��ͬ���칹�壬�����к���2��ȡ��������ȡ������ͬһ�������ϣ�X��NaOH��Һ����ȫˮ���������ˮ�����ĺ˴Ź���������5���塣д��X���ܵĽṹ��ʽ�� ����дһ�֣���
����������X��C��ͬ���칹�壬�����к���2��ȡ��������ȡ������ͬһ�������ϣ�X��NaOH��Һ����ȫˮ���������ˮ�����ĺ˴Ź���������5���塣д��X���ܵĽṹ��ʽ�� ����дһ�֣���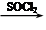 RCOCl����������֪ʶ����������Ϣ��д���Ա�������Ϊԭ���Ʊ�
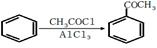
RCOCl����������֪ʶ����������Ϣ��д���Ա�������Ϊԭ���Ʊ�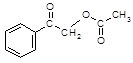 �ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£� CH3CH2Br
CH3CH2Br CH3CH2OH
CH3CH2OH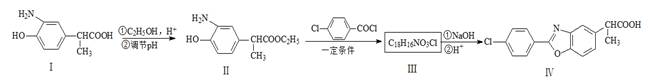 ��ش��������⣺
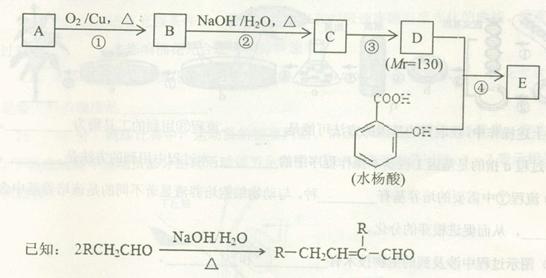
��ش��������⣺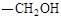 ����A�Ľṹ��ʽ�� ��A�ڴ�����������ͨ���ļ��γɸ߾����д���÷�Ӧ�Ļ�ѧ����ʽ ��
����A�Ľṹ��ʽ�� ��A�ڴ�����������ͨ���ļ��γɸ߾����д���÷�Ӧ�Ļ�ѧ����ʽ ��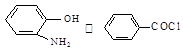 ��һ�������·������ƻ������ת��Ϊ��ķ�Ӧ����д���÷�Ӧ�Ļ�ѧ����ʽ ��
��һ�������·������ƻ������ת��Ϊ��ķ�Ӧ����д���÷�Ӧ�Ļ�ѧ����ʽ ��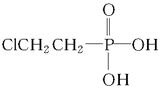 )��D�������������»Ỻ���ͷų�A���ϳ�D��һ�ַ����������ʼ��ת����ϵ����ͼ��ʾ��
)��D�������������»Ỻ���ͷų�A���ϳ�D��һ�ַ����������ʼ��ת����ϵ����ͼ��ʾ��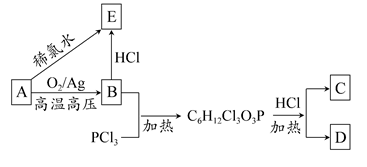
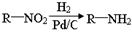
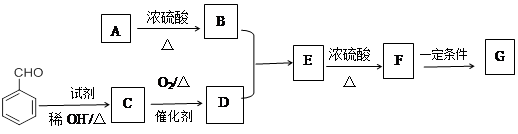
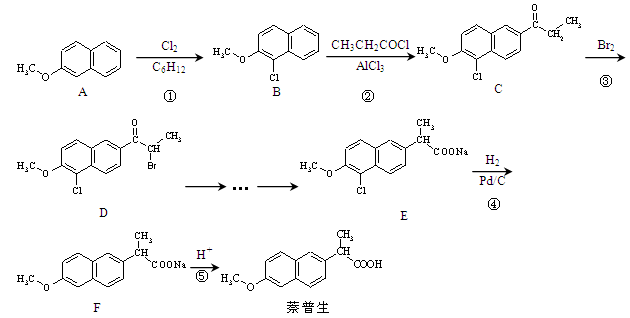
 ��CH3CH2COOHΪԭ�Ϻϳ�
��CH3CH2COOHΪԭ�Ϻϳ� �ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ����
�ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ����