��Ŀ����
�л���Aֻ��C��H��O����Ԫ�أ�����Է�������Ϊ76��Ϊ�ⶨA �Ļ�ѧʽ��ȡ3.8 �˵�A�����ܱ���������ȫȼ�գ���ȼ�ղ���ͨ��ʢ��Ũ������Լ�ƿ���Լ�ƿ����3.6 �ˣ�����ͨ��ʢ�б�������������Һ���Լ�ƿ���Լ�ƿ����6.6�ˣ�
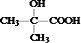
��A�ķ���ʽΪ______��
�ں��������ʾA������ֻ�С�C-C��������C-H�����͡�O-H�����������գ��ɾݴ��ƶ�A���ܵĽṹ��ʽΪ������֪��ͬһ��̼ԭ�������������ǻ��Dz��ȶ��Ľṹ��______��______��
����A�ĺ˴Ź�������ֻ���������շ壬��д��A�����������Ʒ�Ӧ�Ļ�ѧ����ʽ��______����д��A�����������ȣ�ͭ����������Ӧ�Ļ�ѧ����ʽ��______��
��A�ķ���ʽΪ______��
�ں��������ʾA������ֻ�С�C-C��������C-H�����͡�O-H�����������գ��ɾݴ��ƶ�A���ܵĽṹ��ʽΪ������֪��ͬһ��̼ԭ�������������ǻ��Dz��ȶ��Ľṹ��______��______��
����A�ĺ˴Ź�������ֻ���������շ壬��д��A�����������Ʒ�Ӧ�Ļ�ѧ����ʽ��______����д��A�����������ȣ�ͭ����������Ӧ�Ļ�ѧ����ʽ��______��
��ͨ��Ũ��������3.6g��ˮ��������ˮ�����ʵ���Ϊ
=0.2mol����n��H��=2��0.2mol=0.4mol��m��H��=0.4mol��1g/mol=0.4g����������������Һ����6.6�������ɶ�����̼��������������̼�����ʵ���Ϊ
=0.15mol����n��C��=0.15mol��m��C��=0.15mol��12g/mol=1.8g�������A��m��O��=3.8g-0.4g-1.8g=1.6g����n��O��=
=0.1mol������n��C����n��H����n��O��=0.15mol��0.4mol��0.1mol=3��8��2�����ʽΪC3H8O2�������Է���������֪���ʽ��Ϊ����ʽ��
�ʴ�Ϊ��C3H8O2��
�ں��������ʾA������ֻ�С�C-C��������C-H�����͡�O-H�����������գ�ͬһ��̼ԭ�������������ǻ����ȶ�
�ʷ��Ͻṹ�������л���A�Ľṹ��ʽΪ��CH3CH��0H��CH2OH CH2��OH��CH2CH2OH��
�ʴ�Ϊ��CH3CH��0H��CH2OH��CH2��OH��CH2CH2OH��
��A�ĺ˴Ź�������ֻ���������շ壬�����к���3��Hԭ�ӣ�����л���A�ĺ������֪��AΪCH2��OH��CH2CH2OH
��A�����������Ʒ�Ӧ�Ļ�ѧ����ʽΪCH2��OH��CH2CH2OH+2Na��NaOCH2CH2CH2ONa+H2����
A�����������ȣ�ͭ����������Ӧ�Ļ�ѧ����ʽ��CH2��OH��CH2CH2OH+O2
OHCCH2CHO+2H2O��
�ʴ�Ϊ��CH2��OH��CH2CH2OH+2Na��NaOCH2CH2CH2ONa+H2����
CH2��OH��CH2CH2OH+O2
OHCCH2CHO+2H2O��
| 3.6g |
| 18g/mol |
| 6.6�� |
| 44g/mol |
| 1.6g |
| 16g/mol |
�ʴ�Ϊ��C3H8O2��
�ں��������ʾA������ֻ�С�C-C��������C-H�����͡�O-H�����������գ�ͬһ��̼ԭ�������������ǻ����ȶ�
�ʷ��Ͻṹ�������л���A�Ľṹ��ʽΪ��CH3CH��0H��CH2OH CH2��OH��CH2CH2OH��
�ʴ�Ϊ��CH3CH��0H��CH2OH��CH2��OH��CH2CH2OH��
��A�ĺ˴Ź�������ֻ���������շ壬�����к���3��Hԭ�ӣ�����л���A�ĺ������֪��AΪCH2��OH��CH2CH2OH
��A�����������Ʒ�Ӧ�Ļ�ѧ����ʽΪCH2��OH��CH2CH2OH+2Na��NaOCH2CH2CH2ONa+H2����
A�����������ȣ�ͭ����������Ӧ�Ļ�ѧ����ʽ��CH2��OH��CH2CH2OH+O2
| Cu |
| �� |
�ʴ�Ϊ��CH2��OH��CH2CH2OH+2Na��NaOCH2CH2CH2ONa+H2����
CH2��OH��CH2CH2OH+O2
| Cu |
| �� |

��ϰ��ϵ�д�
�����Ŀ
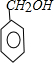
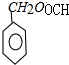 +H2O
+H2O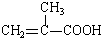
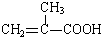 +HO-CH2CH2-OH
+HO-CH2CH2-OH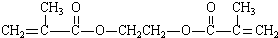 +2H2O
+2H2O
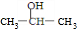 +O2
+O2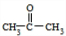 +2H2O��
+2H2O��