��Ŀ����
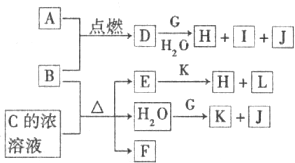
A��GΪ��ѧ�����Ļ��������֮��������ͼ��ʾ��ת����ϵ(��Ӧ���������ֲ�������ȥ)��A����ɫ���壬��H��C��O��Cu����Ԫ�ء�������DΪ��ɫ��ζ���壬BΪ��ɫ��ĩ��E�ܷ���������Ӧ����ش�

(1)D��G��Ӧ�Ļ�ѧ����ʽΪ___________________��
(2)F��һ�����еĹ����ŵ�����Ϊ___________________��
(3)ij����С��ͬѧ���������ʵ��װ�ã�ͨ���ⶨijЩװ�����Լ��������仯��̽��A�и�Ԫ�ص�������ϵ��

��Ϊʹ����ȷ�����貹��װ�ã������ڷ����ڻ��װ��ͼ��д���Լ����ơ�
����װ���й��������Ŀ����______________________________��
��װ����ҩƷ������Ϊ____________________��ʵ��ʱ����ҩƷδ�����Ա仯��֤��________________________________________��
������ж�A����ȫ�ֽ⣿
____________________________________________________________________��
�ܸ���ȷ�IJⶨ�ó��������ݣ�A���Ⱥ���ȫ�ֽ⣬������
(1)2Na2O2+2CO2![]() 2Na2CO3+O2
2Na2CO3+O2
(2)�ǻ�
(3)��
 ��
��![]()
�ڽ�A�ֽ������ˮ��������ʢ��Ũ�����ϴ��ƿ�� ��ˮ����ͭ A�ֽ������ˮ����ȫ����Ũ��������
���������μ��ȡ���������ȴ��������װ�õ����������������0.1 g
��CuCO3��2Cu(OH)2��Cu3(OH)4CO3
����������ɫ������Ԫ�ؿ�֪AΪCu2(OH)2CO3��BΪCuO��D��CO2��D��G��Ӧ����O2����GΪNa2O2��E�ܷ���������Ӧ��˵��F�к��д��ǻ�.(3)��ʵ��Ҫ�ⶨA�ֽ�����ˮ�����������Է�����Ӧ����װ��Ũ�����ϴ��ƿ��װ�м�ʯ�ҵĸ����.����װ���й��������Ŀ���ǽ�A�ֽ������ˮ����ȫ���������У���A�ֽ�����CuO![]() 0.075 mol������H2O
0.075 mol������H2O![]() =0.05 mol������Cu(OH)2��CuCO3�����ʵ����ı�ֵΪ0.05��(0.075-0.05)=2��1��A�Ļ�ѧʽΪ2Cu(OH)2��CuCO3.
=0.05 mol������Cu(OH)2��CuCO3�����ʵ����ı�ֵΪ0.05��(0.075-0.05)=2��1��A�Ļ�ѧʽΪ2Cu(OH)2��CuCO3.

| |||||||||||||||||||

 ��Ч���֣���
��Ч���֣���