ЬтФПФкШн
ЁОЬтФПЁПгаAЁЂBЁЂCЁЂDЁЂEЁЂFЁЂGЁЂLАЫжждЊЫиЃЌЪдАДЯТЪіЫљИјЕФЬѕМўЭЦЖЯЃК
ЂйAЁЂBЁЂCЪЧЭЌвЛжмЦкЕФН№ЪєдЊЫиЃЌвбжЊдзгКЫЭтга3ИіЕчзгВуЃЌAЕФдзгАыОЖдкЫљЪєжмЦкжазюДѓЧвдзгАыОЖA>B>CЃЛ
ЂкDЁЂEЪЧЗЧН№ЪєдЊЫиЃЌЫќУЧИњЧтЛЏКЯПЩЩњГЩЦјЬЌЧтЛЏЮяHDКЭHEЃЌдкЪвЮТЪБЃЌDЕФЕЅжЪЪЧвКЬхЃЌEЕФЕЅжЪЪЧЙЬЬхЃЛ
ЂлFЕФЕЅжЪдкГЃЮТЯТЪЧЦјЬхЃЌаджЪКмЮШЖЈЃЌЪЧГ§ЧтЭтзюЧсЕФЦјЬхЃЛ
ЂмGЪЧГ§ЧтЭтдзгАыОЖзюаЁЕФдЊЫиЁЃ
ЃЈ1ЃЉAЕФУћГЦЪЧ_______ЃЌBЮЛгкжмЦкБэжаЕк_______жмЦкЕк_______зхЁЃ
ЃЈ2ЃЉEЕЅжЪЕФбеЩЋЪЧ_______________ЁЃ
ЃЈ3ЃЉAдЊЫигыDдЊЫиаЮГЩЛЏКЯЮяЕФЕчзгЪНЪЧ___________________________ЁЃ
ЃЈ4ЃЉGЕФЕЅжЪгыЫЎЗДгІЕФЛЏбЇЗНГЬЪНЪЧ______________________________________ЁЃ
ЃЈ5ЃЉFЕФдЊЫиЗћКХЪЧ_____________ЁЃ
ЃЈ6ЃЉдкЩЯЪіЦпжждЊЫижаЃЌзюИпМлбѕЛЏЮяЖдгІЕФЫЎЛЏЮяМюадзюЧПЕФЛЏбЇЪНЪЧ___________ЃЌЫсадзюЧПЕФЛЏбЇЪНЪЧ_____________ЃЌЦјЬЌЧтЛЏЮязюЮШЖЈЕФЛЏбЇЪНЪЧ_____________ЁЃ
ЃЈ7ЃЉНЋCЕФбѕЛЏЮяЖдгІЕФЫЎЛЏЮяЭЖШыЕНAЕФбѕЛЏЮяЖдгІЕФЫЎЛЏЮяжаЗДгІЕФРызгЗНГЬЪНЪЧ______________ЁЃ
ЃЈ8ЃЉLЕФзюМђЕЅЦјЬЌЧтЛЏЮяМзЕФЫЎШмвКЯдМюадЁЃдкЮЂЕчзгЙЄвЕжаЃЌМзЕФЫЎШмвКПЩзїПЬЪДМСH2O2ЕФЧхГ§МСЃЌЫљЗЂЩњЗДгІЕФВњЮяВЛЮлШОЛЗОГЃЌЦфЛЏбЇЗНГЬЪНЮЊ____________________________ЁЃ
ЁОД№АИЁПФЦ Ш§ ЂђA зЯКкЩЋ NaЃЋ![]() Ѓ 2F2ЃЋ2H2O===4HFЃЋO2 He NaOH HBrO4 HF Al(OH)3ЃЋOHЃ===AlOЃЋ2H2O 2NH3ЁЄH2OЃЋ3H2O2===N2ЁќЃЋ8H2O
Ѓ 2F2ЃЋ2H2O===4HFЃЋO2 He NaOH HBrO4 HF Al(OH)3ЃЋOHЃ===AlOЃЋ2H2O 2NH3ЁЄH2OЃЋ3H2O2===N2ЁќЃЋ8H2O
ЁОНтЮіЁП
гЩаХЯЂЂйжаAЁЂBЁЂCЕФКЫЭтЕчзгВуЪ§жЊОљЮЊЕкШ§жмЦкН№ЪєдЊЫиЃЌНсКЯдзгАыОЖПЩжЊAЁЂBЁЂCЗжБ№ЮЊNaЁЂMgЁЂAlЃЛгЩаХЯЂЂкжаЧтЛЏЮяЕФЛЏбЇЪНжЊDЁЂEЮЊЂїAзхдЊЫиЃЌдкЪвЮТЪБЃЌDЕФЕЅжЪЪЧвКЬхЃЌEЕФЕЅжЪЪЧЙЬЬхЃЌ дђDЮЊфхдЊЫиЁЂEЮЊЕтдЊЫиЃЛЂлFЕФЕЅжЪдкГЃЮТЯТЪЧЦјЬхЃЌаджЪКмЮШЖЈЃЌЪЧГ§ЧтЭтзюЧсЕФЦјЬхЃЌдђFЮЊHeЃЛЂмGЪЧГ§ЧтЭтдзгАыОЖзюаЁЕФдЊЫиЃЌдђGЮЊFЁЃЃЈ1ЃЉAЕФУћГЦЪЧФЦЃЌBЮЊУОЃЌЮЛгкжмЦкБэжаЕкШ§жмЦкЕкЂђAзхЃЛЃЈ2ЃЉEЮЊЕтЃЌЕЅжЪЕФбеЩЋЪЧзЯКкЩЋЃЛЃЈ3ЃЉAдЊЫигыDдЊЫиаЮГЩЛЏКЯЮяфхЛЏФЦЕФЕчзгЪНЪЧNaЃЋ![]() ЃЃЛЃЈ4ЃЉGЕФЕЅжЪF2гыЫЎЗДгІЕФЛЏбЇЗНГЬЪНЪЧ2F2ЃЋ2H2O=4HFЃЋO2ЃЛЃЈ5ЃЉFЕФдЊЫиЗћКХЪЧHeЃЛЃЈ6ЃЉдкЩЯЪіЦпжждЊЫижаЃЌзюИпМлбѕЛЏЮяЖдгІЕФЫЎЛЏЮяМюадзюЧПЕФЛЏбЇЪНЪЧNaOHЃЌЫсадзюЧПЕФЛЏбЇЪНЪЧHBrO4ЃЌЦјЬЌЧтЛЏЮязюЮШЖЈЕФЛЏбЇЪНЪЧHFЃЛЃЈ7ЃЉНЋCЕФбѕЛЏЮяЖдгІЕФЫЎЛЏЮяЧтбѕЛЏТСЭЖШыЕНAЕФбѕЛЏЮяЖдгІЕФЫЎЛЏЮяЧтбѕЛЏФЦжаЗДгІЕФРызгЗНГЬЪНЪЧAl(OH)3ЃЋOHЃ===AlOЃЋ2H2OЃЛЃЈ8ЃЉLЕФзюМђЕЅЦјЬЌЧтЛЏЮяМзЕФЫЎШмвКЯдМюадЃЌдђМзЮЊАБЦјЁЃдкЮЂЕчзгЙЄвЕжаЃЌМзЕФЫЎШмвКПЩзїПЬЪДМСH2O2ЕФЧхГ§МСЃЌЫљЗЂЩњЗДгІЕФВњЮяВЛЮлШОЛЗОГЃЌЦфЛЏбЇЗНГЬЪНЮЊ2NH3ЁЄH2OЃЋ3H2O2===N2ЁќЃЋ8H2OЁЃ
ЃЃЛЃЈ4ЃЉGЕФЕЅжЪF2гыЫЎЗДгІЕФЛЏбЇЗНГЬЪНЪЧ2F2ЃЋ2H2O=4HFЃЋO2ЃЛЃЈ5ЃЉFЕФдЊЫиЗћКХЪЧHeЃЛЃЈ6ЃЉдкЩЯЪіЦпжждЊЫижаЃЌзюИпМлбѕЛЏЮяЖдгІЕФЫЎЛЏЮяМюадзюЧПЕФЛЏбЇЪНЪЧNaOHЃЌЫсадзюЧПЕФЛЏбЇЪНЪЧHBrO4ЃЌЦјЬЌЧтЛЏЮязюЮШЖЈЕФЛЏбЇЪНЪЧHFЃЛЃЈ7ЃЉНЋCЕФбѕЛЏЮяЖдгІЕФЫЎЛЏЮяЧтбѕЛЏТСЭЖШыЕНAЕФбѕЛЏЮяЖдгІЕФЫЎЛЏЮяЧтбѕЛЏФЦжаЗДгІЕФРызгЗНГЬЪНЪЧAl(OH)3ЃЋOHЃ===AlOЃЋ2H2OЃЛЃЈ8ЃЉLЕФзюМђЕЅЦјЬЌЧтЛЏЮяМзЕФЫЎШмвКЯдМюадЃЌдђМзЮЊАБЦјЁЃдкЮЂЕчзгЙЄвЕжаЃЌМзЕФЫЎШмвКПЩзїПЬЪДМСH2O2ЕФЧхГ§МСЃЌЫљЗЂЩњЗДгІЕФВњЮяВЛЮлШОЛЗОГЃЌЦфЛЏбЇЗНГЬЪНЮЊ2NH3ЁЄH2OЃЋ3H2O2===N2ЁќЃЋ8H2OЁЃ

ЁОЬтФПЁПФГбаОПадбЇЯАаЁзщгћбаОПгАЯьаПКЭЯЁСђЫсЗДгІЫйТЪЕФЭтНчЬѕМўЃЌЯТБэЪЧЦфЪЕбщЩшМЦЕФгаЙиЪ§ОнЃК
ЪЕбщ ађКХ | аПЕФжЪСП/g | аПЕФзДЬЌ | c(H2SO4) /molЁЄLЃ1 | V(H2SO4) /mL | ЗДгІЧАШм вКЕФЮТЖШ/Ёц | ЬэМгМС |
1 | 0.65 | СЃзД | 0.5 | 50 | 20 | Юо |
2 | 0.65 | ЗлФЉ | 0.5 | 50 | 20 | Юо |
3 | 0.65 | СЃзД | 0.5 | 50 | 20 | 2ЕЮCuSO4ШмвК |
4 | 0.65 | ЗлФЉ | 0.8 | 50 | 20 | Юо |
5 | 0.65 | ЗлФЉ | 0.8 | 50 | 35 | 2ЕЮCuSO4ШмвК |
ЃЈ1ЃЉдкДЫ5зщЪЕбщжаЃЌХаЖЯаПКЭЯЁСђЫсЗДгІЫйТЪДѓаЁЃЌзюМђЕЅЕФЗНЗЈПЩЭЈЙ§ЖЈСПВтЖЈ______________________НјааХаЖЯЃЌЦфЫйТЪзюПьЕФЪЕбщЪЧ________(ЬюЪЕбщађКХ)ЁЃ
ЃЈ2ЃЉЖдаПКЭЯЁСђЫсЗДгІЃЌЪЕбщ1КЭ2БэУїЃЌ________ЖдЗДгІЫйТЪгагАЯьЃЛЪЕбщ1КЭ3БэУїЃЌ________ЖдЗДгІЫйТЪгагАЯьЁЃ
ЃЈ3ЃЉНјааЪЕбщ2ЪБЃЌаЁзщЭЌбЇИљОнЪЕбщЙ§ГЬЛцжЦЕФБъзМзДПіЯТЕФЦјЬхЬхЛ§VгыЪБМф tЕФЭМЯёШчЯТЭМЫљЪОЁЃ
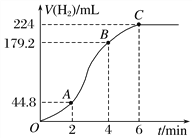
ЂйдкOAЁЂABЁЂBCШ§ЖЮжаЗДгІЫйТЪзюПьЕФЪЧ________ЃЌдвђЪЧ______________________ЁЃ
Ђк2ЁЋ4 minФквдСђЫсЕФХЈЖШБфЛЏБэЪОЕФЗДгІЫйТЪ(МйЩшШмвКЕФЬхЛ§ВЛБф)ЮЊ________________________________________________________________________ЁЃ
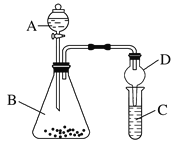
ЃЈ4ЃЉРћгУШчЭМ2зАжУбщжЄЗЧН№ЪєадЃКCЃОSiЃЌBжаМгNa2CO3ЃЌCжаМгNa2SiO3ШмвКЃЌAжагІИУМгШы__________________________ЃЌCжаЗДгІЕФЛЏбЇЗНГЬЪНЃК________________________ЃЌDзАжУЕФзїгУЪЧ_______________________________________ЁЃ
