��Ŀ����
ij��̬��A�ڱ�״���µ��ܶ�Ϊ1.25 g/L�������������������һ�����ҵ�ʯ�ͻ�����չˮƽ��B��D���������г������л��D�ܸ�̼�����Ʒ�Ӧ��F����ζ������֮���ת����ϵ������ʾ
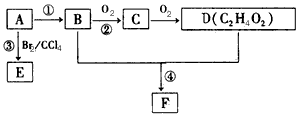
(1)A�ĽṹʽΪ________��B�й����ŵĵ���ʽΪ________��D�й����ŵ�����Ϊ________��
(2)��Ӧ�ٵķ�Ӧ������_______����Ӧ�۵Ļ�ѧ����ʽΪ___________________
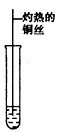
(3)��Ӧ����Cu�������������½��У���ʵ��IJ����ǽ�������ͭ˿���ھƾ����ϼ��ȣ���ͭ˿��Ϊ��ɫʱ��Ѹ�ٽ�����뵽װ��B���Թ��У���ͼ��ʾ�����ظ�����2-3�Ρ��÷�Ӧ�Ļ�ѧ����ʽΪ____________________
(2)��Ӧ�ٵķ�Ӧ������_______����Ӧ�۵Ļ�ѧ����ʽΪ___________________
(3)��Ӧ����Cu�������������½��У���ʵ��IJ����ǽ�������ͭ˿���ھƾ����ϼ��ȣ���ͭ˿��Ϊ��ɫʱ��Ѹ�ٽ�����뵽װ��B���Թ��У���ͼ��ʾ�����ظ�����2-3�Ρ��÷�Ӧ�Ļ�ѧ����ʽΪ____________________
(4)D��̼��������Һ��Ӧ�����ӷ���ʽΪ____________________
(5)B��D��Ũ����������·�����Ӧ�ܣ�ʵ��װ����ͼ��ʾ
(5)B��D��Ũ����������·�����Ӧ�ܣ�ʵ��װ����ͼ��ʾ
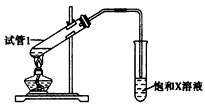
�Թ���װ��ҩƷ����ȡ�ͼ��X�Ļ�ѧʽΪ________����������___________________���Թ��з�Ӧ�Ļ�ѧ����ʽΪ___________________
(1)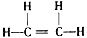 ��
��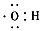 ���Ȼ�
���Ȼ�
(2)�ӳɷ�Ӧ��CH2=CH2+Br2��CH2BrCH2Br
(3)2CH3CH2OH+O2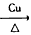 2CH3CHO+2H2O ����2Cu+O2
2CH3CHO+2H2O ����2Cu+O2 2CuO��CH3CH2OH+CuO
2CuO��CH3CH2OH+CuO CH3CHO+Cu+H2O)
CH3CHO+Cu+H2O)
(4)CH3COOH+HCO3-��CH3COO-+H2O+CO2��
(5)Na2CO3���ܽ��Ҵ����������ᣬ���������������ķֲ�������
CH3CH2OH+CH3COOH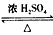 CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O
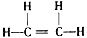 ��
��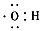 ���Ȼ�
���Ȼ�(2)�ӳɷ�Ӧ��CH2=CH2+Br2��CH2BrCH2Br
(3)2CH3CH2OH+O2
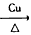 2CH3CHO+2H2O ����2Cu+O2
2CH3CHO+2H2O ����2Cu+O2 2CuO��CH3CH2OH+CuO
2CuO��CH3CH2OH+CuO CH3CHO+Cu+H2O)
CH3CHO+Cu+H2O)(4)CH3COOH+HCO3-��CH3COO-+H2O+CO2��
(5)Na2CO3���ܽ��Ҵ����������ᣬ���������������ķֲ�������
CH3CH2OH+CH3COOH
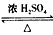 CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O 
��ϰ��ϵ�д�
�����Ŀ















