��Ŀ����
ij��̬��A�ڱ�״���µ��ܶ�Ϊ1.25g/L�������������������һ�����ҵ�ʯ�ͻ�����չˮƽ��B��D���������г������л��D�ܸ�̼�����Ʒ�Ӧ��F����ζ������֮���ת����ϵ����ͼ��ʾ��


 ��1��A�ĽṹʽΪ ��B�й����ŵĵ���ʽΪ
��
��1��A�ĽṹʽΪ ��B�й����ŵĵ���ʽΪ
��
 D�й����ŵ�����Ϊ
��
D�й����ŵ�����Ϊ
��
 ��2����Ӧ�ٵķ�Ӧ������ ��
��2����Ӧ�ٵķ�Ӧ������ ��
��Ӧ�۵Ļ�ѧ����ʽΪ ��
��

 ��3����Ӧ����Cu�������������½��У���ʵ��IJ����ǽ�������ͭ˿���ھƾ����ϼ��ȣ���ͭ˿��Ϊ��ɫʱ��Ѹ�ٽ�����뵽װ��B���Թ��У���ͼ��ʾ�����ظ�����2��3�Ρ��÷�Ӧ�Ļ�ѧ����ʽΪ
��
��3����Ӧ����Cu�������������½��У���ʵ��IJ����ǽ�������ͭ˿���ھƾ����ϼ��ȣ���ͭ˿��Ϊ��ɫʱ��Ѹ�ٽ�����뵽װ��B���Թ��У���ͼ��ʾ�����ظ�����2��3�Ρ��÷�Ӧ�Ļ�ѧ����ʽΪ
��
 ��4��D��̼��������Һ��Ӧ�����ӷ���ʽΪ
��
��4��D��̼��������Һ��Ӧ�����ӷ���ʽΪ
��
 ��5��B��D��Ũ�����������ʵ�ַ�Ӧ�ܣ�ʵ��װ������ͼ��ʾ��
��5��B��D��Ũ�����������ʵ�ַ�Ӧ�ܣ�ʵ��װ������ͼ��ʾ��

 �Թ�1��װ��ҩƷ����ȡ�ͼ��X�Ļ�ѧʽΪ
��
�Թ�1��װ��ҩƷ����ȡ�ͼ��X�Ļ�ѧʽΪ
��

 ��������
��
��������
��
 �Թ�1��Ӧ�Ļ�ѧ����ʽΪ
��
�Թ�1��Ӧ�Ļ�ѧ����ʽΪ
��
��1��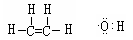 �Ȼ���
�Ȼ���

 ��2���ӳɣ�CH2=CH2
+ Br2��CH2BrCH2Br
��2���ӳɣ�CH2=CH2
+ Br2��CH2BrCH2Br
 ��3��
��3��

 ��4��CH3COOH+HCO3-��CH3COO-+H2O+CO2��
��4��CH3COOH+HCO3-��CH3COO-+H2O+CO2��
 ��5��Na2CO3���ܽ��Ҵ����������ᣬ���������������ֲ㡣
��5��Na2CO3���ܽ��Ҵ����������ᣬ���������������ֲ㡣




����������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�










