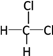
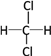
��Ŀ����
����Ŀ��2016�궬�����������������Ű�ҹ��ж������������У�����β����ȼúβ������ɿ�����Ⱦ��ԭ��֮һ��
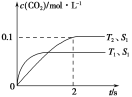
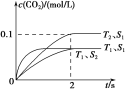
(1)����β����������Ҫԭ��Ϊ��2NO(g)��2CO(g) ![]() 2CO2(g)��N2(g)�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯���ߣ�����ͼ��ʾ��
2CO2(g)��N2(g)�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯���ߣ�����ͼ��ʾ��
�ݴ��жϣ�
�ٸ÷�Ӧ�Ħ�H________0(�>����<��)��
����T2�¶��£�0��2 s�ڵ�ƽ����Ӧ����v(N2)________��
�۵��������������һ��ʱ�����������������ѧ��Ӧ���ʡ��������ı����S1>S2������ͼ�л���c(CO2)��T1��S2�����´ﵽƽ������еı仯���ߡ�
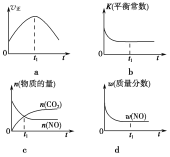
�����÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У�����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬����________(�����)��
(2)ֱ���ŷ�úȼ�ղ������������������صĻ������⡣
��úȼ�ղ����������������������CH4����ԭNOx�������������������Ⱦ��
���磺
CH4(g)��2NO2(g)===N2(g)��CO2(g)��2H2O(g) ��H1����867 kJ/mol
2NO2(g) ![]() N2O4(g) ��H2����56.9 kJ/mol
N2O4(g) ��H2����56.9 kJ/mol
д��CH4(g)����ԭN2O4(g)����N2(g)��H2O(g)���Ȼ�ѧ����ʽ��_______________________
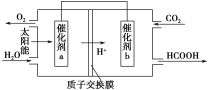
�ڽ�ȼú�����Ķ�����̼�������ã��ɴﵽ��̼�ŷŵ�Ŀ�ġ���ͼ��ͨ���˹�������ã���CO2��H2OΪԭ���Ʊ�HCOOH��O2��ԭ��ʾ��ͼ������b���淢���ĵ缫��ӦʽΪ__________________________��
���𰸡�(1)��< ��0.025 mol��(L��s)��1
��
��bd
(2)��CH4(g)��N2O4(g)===N2(g)��CO2(g)��2H2O(g)
��H����810.1 kJ/mol
��CO2��2H����2e��===HCOOH
��������(1)�ٸ���ͼ���ȹ���ƽ��ֵ��T1����T2�������¶ȣ�������̼��Ũ�Ƚ��ͣ�ƽ�����淴Ӧ�����ƶ���˵������Ӧ�Ƿ��ȷ�Ӧ������H<0�����ȸ���ͼ����������̼�ķ�Ӧ���ʣ�v(CO2)��![]() ��0.05 mol��(L��s)��1��ͬһ��ѧ��Ӧ��ͬһʱ����ڣ������ʵķ�Ӧ����֮�ȵ��ڼ�����֮�ȣ�����v(N2)��0.025 mol��(L��s)��1�����¶�Խ�߷�Ӧ����Խ�����Ӵ����Խ��Ӧ����Խ��Ӧ����ƽ���ʱ��Խ�̡��ܷ�Ӧ�ﵽƽ��״̬ʱ�������ʵķ�Ӧ���ʲ��ٱ仯����a���÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У��淴Ӧ�����¶����ߣ����¶Ȳ���ʱ����ѧƽ�ⳣ�����䣬��b��ȷ��������̼��һ�����������ʵ������ʱ���÷�Ӧ��һ���ﵽƽ��״̬����c����Ӧ�ﵽƽ��״̬ʱ�������ʵ������������ٷ����仯����d��ȷ��
��0.05 mol��(L��s)��1��ͬһ��ѧ��Ӧ��ͬһʱ����ڣ������ʵķ�Ӧ����֮�ȵ��ڼ�����֮�ȣ�����v(N2)��0.025 mol��(L��s)��1�����¶�Խ�߷�Ӧ����Խ�����Ӵ����Խ��Ӧ����Խ��Ӧ����ƽ���ʱ��Խ�̡��ܷ�Ӧ�ﵽƽ��״̬ʱ�������ʵķ�Ӧ���ʲ��ٱ仯����a���÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У��淴Ӧ�����¶����ߣ����¶Ȳ���ʱ����ѧƽ�ⳣ�����䣬��b��ȷ��������̼��һ�����������ʵ������ʱ���÷�Ӧ��һ���ﵽƽ��״̬����c����Ӧ�ﵽƽ��״̬ʱ�������ʵ������������ٷ����仯����d��ȷ��
(2)�ٽ���һ������ʽ���ڶ�������ʽ�ã�CH4(g)��N2O4(g)===N2(g)��CO2(g)��2H2O(g)
��H����810.1 kJ/mol���ڴ���b�����϶�����̼�õ��Ӻ������ӷ�Ӧ���ɼ��ᣬ���Է����ĵ缫��ӦʽΪ��CO2��2H����2e��===HCOOH��

 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д� �����ƻ���ĩ��̶�100��ϵ�д�
�����ƻ���ĩ��̶�100��ϵ�д� �ܿ���ȫ��100��ϵ�д�
�ܿ���ȫ��100��ϵ�д�