��Ŀ����
���и���Һ�У��������ʵ���Ũ�ȹ�ϵ��ȷ����
| A����NaHA��Һ��һ���У�c(Na��)��c(H��)��c(HA��)��c(OH��)��c(A2-) |
| B����0.1mol��L��1Na2CO3��Һ�У�c(OH��)==c(HCO3��)��c(H+)+ c(H2CO3) |
| C��pH>7��NaCN��HCN�Ļ����Һ��һ���У�c(Na��)��c(CN��) >c(OH��) >c(H+) |
| D��pH>7��CH3COONa��NaOH�Ļ����Һ��һ���У�c(Na+)>c(CH3COO��) >c(OH��) >c(H+) |
C
��

��ϰ��ϵ�д�
�����Ŀ
 )= ��
)= ��
 ��H+��
��H+�� ��Na+�������ӣ�ijͬѧ�Ʋ�������Ũ�ȴ�С˳�����������ֹ�ϵ��
��Na+�������ӣ�ijͬѧ�Ʋ�������Ũ�ȴ�С˳�����������ֹ�ϵ��



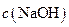
 (�<������>����=��)�����ǰ
(�<������>����=��)�����ǰ ��Һ�е�
��Һ�е�
 ��Һ�е�
��Һ�е� (�<������>����=��)��
(�<������>����=��)�� (HCO3��)+c(CO32��)
(HCO3��)+c(CO32��)
 ��
�� ��
�� ��
��
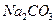 ��
�� ��
��
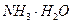 ��
��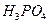 ��
�� ��
��