��Ŀ����
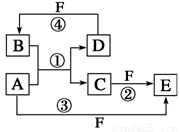
(10��)A��B��C��D��E��F�������ʵ�ת����ϵ��ͼ��ʾ(��Ӧ�����Ͳ��ֲ���δ���)��
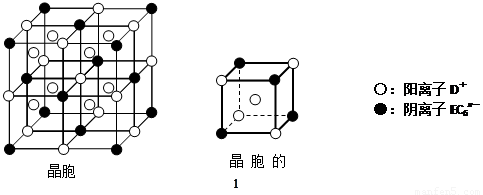
(1)��AΪ�����ڽ������ʣ�DΪ�����ڷǽ������ʣ�������Ԫ�ص�ԭ������A��D��2��������Ԫ�ص�ԭ������������D��A��2����F��Ũ��Һ��A��D��Ӧ���к���ɫ�������ɣ���C�ĵ���ʽΪ ����Ӧ�ܵĻ�ѧ����ʽΪ_______________________________________��
(2)��A�dz����ı�۽����ĵ��ʣ�D��F����̬���ʣ��ҷ�Ӧ����ˮ��Һ�н��С���д����ˮ��Һ�н��еķ�Ӧ�ڵ����ӷ���ʽ ��
��֪����������D��F��Ӧ����B��д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
(3)��A��D��F���Ƕ����ڷǽ���Ԫ�ص��ʣ���A��D����Ԫ��ͬ���壬A��F����Ԫ��ͬ���ڣ���Ӧ�ٵĻ�ѧ����ʽΪ ��
(1)
(2) 2Fe2����Cl2 === 2Fe3����2Cl��
(3)
����:��1������ɫ������NO2������F��Ũ���ᡣ�����û���Ӧ���ڳ��������û��ǽ����ķ�Ӧ�У����˻��ý����û������⣬����þ��CO2��ȼ�����ɵ���̼������ʽΪ2MgO��CO22MgO��C����A��þ��B��CO2��C��MgO��D��̼��
��2�������ı�۽����ĵ������������ݿ�ͼ��֪A��C��E�о�������Ԫ�أ��Ҽ�̬��ͬ������FӦ�Ǿ��������Ե���������A��B��C��D��E��F�ֱ�ΪFe��HCl��FeCl2��H2��FeCl3��Cl2��
��3�����ݿ�ͼ��֪A��C��E�о�����AԪ�أ��Ҽ�̬��ͬ�����Կ�����̼Ԫ�ء�����Ϊ�ڸ�����̼�����û�SiO2�еĹ裬����ʽΪSiO2��2CSi��2CO������A��B��C��D��E��F�ֱ�ΪC��SiO2��CO��Si��CO2��O2��

 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�