��Ŀ����
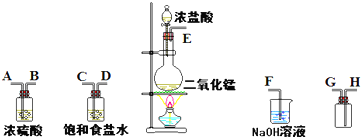
��ʵ�������ö������̸�Ũ���ᷴӦ�Ʊ����﴿�������������д�ʵ�飬����������ͼ2-28��ʾ��
ͼ2-28
��1������������������ȷ˳���ǣ�����ӿڴ�����ĸ����
_________��_________��_________��_________��_________��_________��_________��_________��
��2����װ���У��ٱ���ʳ��ˮ���������_________________________________________��
��Ũ�������������________________________________________________��
��3����ѧʵ���м����Ƿ���Cl2��������ʪ��ĵ���KI��ֽ�������Cl2�������ɹ۲쵽��������___________________��д����Ӧ����ʽ��___________________________________��ijͬѧ����ֽ��ʱ����ڲ���Cl2�ĵ��ܿڣ��ڿ����������������ֽ�ֱ�Ϊ��ɫ��д����ʱ������������ԭ����ʽ��_______________________________________��
��4��д�����л�ѧ��Ӧ�����ӷ���ʽ��
�����巢��װ���н��еķ�Ӧ��__________________________________________��
��β������װ���н��еķ�Ӧ��__________________________________________��
��1��E C D A B H G F
��2���ٳ�ȥ���������е�HCl���� �ڸ�������
��3����ֽ����ɫ 2KI+Cl2![]() 2KCl+I2 I2+5Cl2+6H2O
2KCl+I2 I2+5Cl2+6H2O![]() 2HIO3+10HCl
2HIO3+10HCl
��4����MnO2+4H++2Cl-![]() Mn2++Cl2��+2H2O
Mn2++Cl2��+2H2O
��2OH-+Cl2![]() Cl-+ClO-+H2O
Cl-+ClO-+H2O



 MnCl2+Cl2+2H2O
MnCl2+Cl2+2H2O
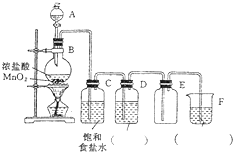 ��ʵ�������ö������̺�Ũ���ᷴӦ�Ʊ������������װ��ͼ��ͼ��
��ʵ�������ö������̺�Ũ���ᷴӦ�Ʊ������������װ��ͼ��ͼ��