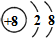��Ŀ����
(һ)������A��B��C��D ����Ԫ�أ�ԭ��������������Aԭ�ӵ����������4�����ӣ�B�������Ӻ�C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ����Ԫ�صĵ��ʷ�Ӧ��������һ��ԭ�Ӹ�����Ϊ1��1���Ҳ�����ˮ�Ĺ���E��D��L�����������K��M�������Ӳ��ϵ�����֮�͡�
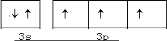
��1��AԪ�ص�����Ϊ ��2��Bԭ�ӵ������ӵĽṹʾ��ͼΪ ��
��3��CԪ��λ��Ԫ�����ڱ��е� ���ڡ��� �塣
(4) D������������������ȣ���������������Ӧ��ˮ���ﻯѧʽΪ�� ��
����������Ԫ�����ڱ�����Ԫ�������ɻش��������⣺
��1��Ԥ��52��Ԫ�������ڱ��е�λ�ã��� ���� �塣
��2����֪��Ϊ�������ڵڢ�A��Ԫ�أ��ݴ��Ʋ��������ܾ��е������ǣ� ��
A����������ϼ�Ϊ��6�ۣ� B����̬�⻯���H2S�ȶ���
C������������ˮ��������Ա��������� D�������ڳ����¿����������ϡ�
��3����֪XΪ��A��Ԫ�أ���ԭ������Ϊa��Y��Xλ��ͬһ���ڣ���Ϊ��A��Ԫ�أ���Y��ԭ������b�� ��д��b��a���п��ܵĹ�ϵ����
(һ) ��1��̼ ��2�� �� ��3��������A (4) H2SO4
�� ��3��������A (4) H2SO4
��������1���� ��A ��2��BD
��3��b�� a+1 b=a+11 b=a+25 ��д��b��a���п��ܵĹ�ϵ��
����