��Ŀ����
�ⶨ����ͭ����(CuSO4��5H2O)��ᾧˮ�ĺ�����ʵ�鲽��Ϊ������ĥ �ڳ�����������װ������������������ �ۼ��� ����ȴ �ݳ��� ���ظ������ݵIJ�����ֱ���������γ��������������0.1 gΪֹ �߸���ʵ�����ݼ�������ͭ������ᾧˮ�ĺ�������������и��⣺
(1)��������������ǯ�����żܡ������ǡ�����������������ҩ�ס�����ͭ������Ʒ��ʵ����Ʒ�����и�ʵ��ʱ��ȱ�ٵ�ʵ����Ʒ��__________________________________________��
(2)��ʵ������һ������Ҫʹ�ø�������ʹ�ø�������Ŀ����ʲô��
��_________________________________________________________________��
(3)ʵ�鲽���Ŀ����____________________________________________________��
(4)��������ȷ��ʵ���õ�����ͭ�����нᾧˮ�ĺ���ƫ�ͣ���ԭ�������(����ѡ��ı��) ____________��
A.������Ʒ�к��м��Ȳ��ӷ�������
B.������Ʒ�к��м����ӷ�������
C.ʵ��ǰ������Ʒ���в���ʧˮ
D.����ǰ���õ�����δ��ȫ����
����:����ĥ��Ҫ�в������������������Ҫ������ƽ��������Ҫ�ƾ��ơ�
���ȵ���ˮCuSO4����ȴ�Ĺ����п������տ����е�ˮ�����ԣ�CuSO4����ȴҪ�ڸ������н��С�
�ظ��ۺ͢ݵIJ���һֱ�����γ����Ľ��������0.1 g�����Ա�֤CuSO4��5H2Oȫ���ֽ⡣
��:(1)�в���������ƽ���ƾ���(���벻��������)
(2)����ȴ��Ŀ���Ƿ�ֹ��ˮ
(3)������Ʒ�нᾧˮ�Ƿ��Ѿ�ȫ����ȥ
(4)AC

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
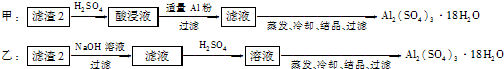
����ѵ��ϵ�д���Ϣʱ�������Ĵ������������Ի��������˼������в��ij��ѧ��ȤС�齫һ����������·������õ���Ҫ��Cu��Al������Fc��Au��Pt�Ƚ����Ļ�������������Ʊ�����ͭ����������������·�ߣ�
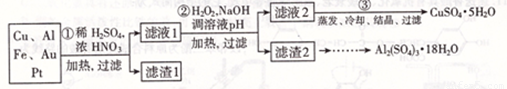
����������������������ʽ����ʱ��Һ��pH���±�
|
������ |
Fe( OH)2 |
Fe( OH)3 |
Al( OH)3 |
Cu( OH)2 |
|
��ʼ���� |
5.8 |
1.1 |
4.0 |
5.4 |
|
��ȫ���� |
8.8 |
3.2 |
5.2 |
6.7 |
��ش��������⣺
��1���ڢٲ�����ǰ�轫�����������з��飬��Ŀ���� ��
��2��ijѧ����Ϊ��H2O2����ŨHNO3���ã������� ��
��д��Cu����H2O2��ϡ��������Һ�����ӷ���ʽ�� ��
��3���ڢڲ���Ӧ����ҺpH���� ��
��4��������2��ȡAl2( SO4)3��18H2O��̽��С����������ַ�����
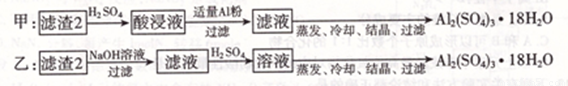
����Ϊ �ַ���Ϊ��ѷ����������� �� ��
��5��Ϊ�˲ⶨ����ͭ����Ĵ��ȣ�ijͬѧȷ��ȡ4.0g��Ʒ����ˮ���l00mL��Һ��ȡl0m��Һ�ڴ�����ƿ�У�������ˮϡ�ͣ�������ҺpH=3��4�����������KI����0.l000mol��L-1Na2S2O3����Һ�ζ����յ㣬������14. 00mL Na2S2O3����Һ�����������з�Ӧ�����ӷ���ʽ���£�
2Cu2+ +4I��=2CuI����ɫ����+I2
2S2O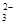 +I2=
2I��+S4O
+I2=
2I��+S4O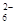
����Ʒ������ͭ�������������Ϊ____ ��
����һλͬѧ���ͨ���ⶨ��Ʒ����������ӵ���Ҳ���������ͭ����Ĵ��ȣ�����ͬѧ��Ϊ�˷��������У������� ��