��Ŀ����
500mLKNO3��Cu(NO3)2�Ļ����Һ��c(NO3-)��6.0mol��L-1����ʯī���缫������Һ����ͨ��һ��ʱ����������ռ���22.4L����(��״��)���ٶ�������Һ�����Ϊ500mL������˵����ȷ����
- A.ԭ�����Һ��c(K+)Ϊ2mol��L-1
- B.�����������й�ת��2mol����
- C.���õ�ͭ�����ʵ���Ϊ0.5mol
- D.������Һ��c(H+)Ϊ2mol��L-1
A
���KNO3��Cu(NO3)2�������缫��Ӧʽ��4OH--4e- 2H2O+O2������O2 22.4 L(��״��������ת�Ƶ�����Ϊ4mol�������缫��Ӧʽ���ȷ�����Cu2++2e-
2H2O+O2������O2 22.4 L(��״��������ת�Ƶ�����Ϊ4mol�������缫��Ӧʽ���ȷ�����Cu2++2e-![]() Cu��������2H++2e-
Cu��������2H++2e-![]() H2������H21mol�����ʽת�Ƶ�����Ϊ2 mol��ǰʽת�Ƶ�����Ϊ(4-2)="2" mol����n(Cu2+)="1" mol,n��Cu(NO3)2��="1" mol���ܹ�n(NO3-)="0.5��6=3" mol,��n(KNO3)=3-n(Cu2+)��2="1" mol��A��c(K+)=
H2������H21mol�����ʽת�Ƶ�����Ϊ2 mol��ǰʽת�Ƶ�����Ϊ(4-2)="2" mol����n(Cu2+)="1" mol,n��Cu(NO3)2��="1" mol���ܹ�n(NO3-)="0.5��6=3" mol,��n(KNO3)=3-n(Cu2+)��2="1" mol��A��c(K+)=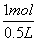 =2mol��L-1;B��ת�Ƶ�����Ϊ4 mol;C��n(Cu)=1mol;D��
=2mol��L-1;B��ת�Ƶ�����Ϊ4 mol;C��n(Cu)=1mol;D��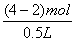 =c(H+),��c(H+)Ϊ4 mol��L-1��
=c(H+),��c(H+)Ϊ4 mol��L-1��
���KNO3��Cu(NO3)2�������缫��Ӧʽ��4OH--4e-
 2H2O+O2������O2 22.4 L(��״��������ת�Ƶ�����Ϊ4mol�������缫��Ӧʽ���ȷ�����Cu2++2e-
2H2O+O2������O2 22.4 L(��״��������ת�Ƶ�����Ϊ4mol�������缫��Ӧʽ���ȷ�����Cu2++2e-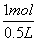 =2mol��L-1;B��ת�Ƶ�����Ϊ4 mol;C��n(Cu)=1mol;D��
=2mol��L-1;B��ת�Ƶ�����Ϊ4 mol;C��n(Cu)=1mol;D��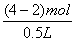 =c(H+),��c(H+)Ϊ4 mol��L-1��
=c(H+),��c(H+)Ϊ4 mol��L-1�� 
��ϰ��ϵ�д�
�����Ŀ
500mlKNO3��Cu��NO3��2�Ļ����Һ��c��NO3-��=6mol��L-1����ʯī���缫������Һ����ͨ��һ��ʱ����������ռ���22.4L���壨��״�������ٶ�������Һ�����Ϊ500ml������˵����ȷ����
| A��ԭ�����Һ��c��K+��=4mol��L-1 |
| B�����õ���Cu�����ʵ���Ϊ0.5mol |
| C��������Һ��c��H+��=2mol��L-1 |
| D����������Һ�м���һ������Cu��OH��2�ɻָ�Ϊԭ��Һ |