ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ25Γφ ±Θ§c(CH3COOH)+c(CH3COO-)=0.1molL-1ΒΡ¥ΉΥαΓΔ¥ΉΥαΡΤΜλΚœ»ή“Κ÷–Θ§c(CH3COOH)ΓΔc(CH3COO-)”κpHΒΡΙΊœΒ»γΆΦΥυ ΨΘ°œ¬Ν––π ω’ΐ»ΖΒΡ «( )
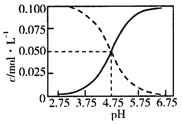
AΘ°25Γφ ±Θ§CH3COOHΒΡΒγάκ≥Θ ΐKΒΡ÷Β5ΓΝ10-2.75
BΘ°ΥφpH‘ω¥σΘ§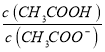 ‘ω¥σ
‘ω¥σ
CΘ°pH=4ΒΡ»ή“Κ÷–Θ§c(CH3COO-)ΘΨc(CH3COOH)ΘΨc(H+)ΘΨc(OH-)
DΘ°pH=5ΒΡ»ή“Κ÷–Θ§c(H+)+c(Na+)+c(CH3COOH)-c(OH-)=0.1molL-1
ΓΨ¥πΑΗΓΩD
ΓΨΫβΈωΓΩ
‘ΧβΖ÷ΈωΘΚΥφΦν–‘‘ω«ΩΘ§»ή“Κ÷–c(CH3COOH)Φθ–ΓΘ§c(CH3COO-)‘ω¥σΘ§–ιœΏ±μ Ψc(CH3COOH)Θ§ ΒœΏ±μ Ψc(CH3COO-)ΓΘAΘ°”…ΆΦΩ…÷ΣΘ§pH=4.75 ±Θ§»ή“Κ÷–c(H+)=10-4.75mol/LΘ§c(CH3COOH)=c(CH3COO-)=0.05mol/LΘ§‘ρCH3COOHΒΡΒγάκ≥Θ ΐK= =c(H+)=10-4.75mol/LΘ§Ι A¥μΈσΘΜBΘ°”…¥ΉΥαΒγάκΤΫΚβ≥Θ ΐΩ…÷ΣΘΚ
=c(H+)=10-4.75mol/LΘ§Ι A¥μΈσΘΜBΘ°”…¥ΉΥαΒγάκΤΫΚβ≥Θ ΐΩ…÷ΣΘΚ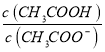 =
=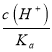 Θ§ΥφpH‘ω¥σΘ§c(H+)Φθ–ΓΘ§‘ρ
Θ§ΥφpH‘ω¥σΘ§c(H+)Φθ–ΓΘ§‘ρ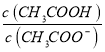 Φθ–ΓΘ§Ι B¥μΈσΘΜCΘ°”…ΆΦΩ…÷ΣΘ§pH=4 ±Θ§c(CH3COOH)ΘΨc(CH3COO-)Θ§Ι C¥μΈσΘΜDΘ°”…ΒγΚ… ΊΚψΩ…÷ΣΘΚc(Na+)+c(H+)=c(CH3COO-)+c(OH-)Θ§Εχ»ή“Κ÷–c(CH3COOH)+c(CH3COO-)=0.1molL-1Θ§ΝΣΝΔΩ…ΒΟc(Na+)+c(H+)+c(CH3COOH)-c(OH-)=0.1molL-1Θ§Ι D’ΐ»ΖΓΘΙ ―ΓDΓΘ
Φθ–ΓΘ§Ι B¥μΈσΘΜCΘ°”…ΆΦΩ…÷ΣΘ§pH=4 ±Θ§c(CH3COOH)ΘΨc(CH3COO-)Θ§Ι C¥μΈσΘΜDΘ°”…ΒγΚ… ΊΚψΩ…÷ΣΘΚc(Na+)+c(H+)=c(CH3COO-)+c(OH-)Θ§Εχ»ή“Κ÷–c(CH3COOH)+c(CH3COO-)=0.1molL-1Θ§ΝΣΝΔΩ…ΒΟc(Na+)+c(H+)+c(CH3COOH)-c(OH-)=0.1molL-1Θ§Ι D’ΐ»ΖΓΘΙ ―ΓDΓΘ