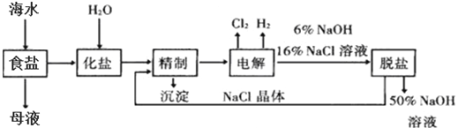
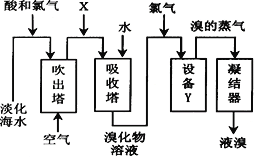
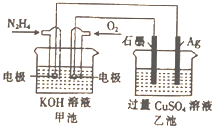
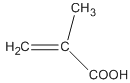
��Ŀ����
����Ŀ��O2��O3����Ԫ�ص����ֵ��ʣ����������ʽ������и��⣺
��1����������O2��O3�������Ӹ�����Ϊ___________��ԭ�Ӹ�����Ϊ____________�����ӵ����ʵ���֮��Ϊ__________��
��2�����µ�ѹ�£��������O2��O3�������Ӹ�����Ϊ___________��ԭ�Ӹ�����Ϊ___________��������Ϊ__________��
��3����NAΪ�����ӵ���������ֵ�����a g�����к��еķ�����Ϊb����c g�����ڱ�״���µ����Լ��__________(�ú�NA��ʽ�ӱ�ʾ)��
��4��ʵ����������2mol��L��1��NaOH��Һ950mL������ʱ���ȡ��NaOH��������_____________��
��5���ڱ�״����15gCO��CO2�Ļ�����壬���Ϊ11.2L��������ƽ��Ħ��������__________��CO2��CO�����֮����____________��
���𰸡�3:2 1:1 3:2 1:1 2:3 2:3 ![]() L 80g 30g/mol 7��1
L 80g 30g/mol 7��1
��������
��1��O2��O3�����ʵ���֮�ȣ��͵��ڷ�����֮�ȣ�������ɷ��ӵ�ԭ��������ԭ����֮�ȣ�
��2����ͬ�����£����������ȵ������ʵ���֮�ȣ����ڷ�����֮�ȣ�
��3���������ʵ�������ع�ʽ���м��㣻
��4��������Һʱ������ƿ��ѡ��Ӧ������������С����ԭ��ѡʵ���ҳ��й�������ƿ������Ӧѡ1000mL������ƿ���Դ˼�������NaOH��������
��5�������ƽ��Ħ������Ϊ��M=![]() ��CO2��CO�����֮�ȵ���CO2��CO�����ʵ���֮�ȣ�
��CO2��CO�����֮�ȵ���CO2��CO�����ʵ���֮�ȣ�
��1����������O2��O3�����ʵ����ֱ�Ϊ��n(O2)=![]() ��n(O3)=
��n(O3)=![]() ����n(O2)��n(O3)=3:2������O2��O3�ķ�����֮��Ϊ3:2��ԭ����֮��Ϊ��
����n(O2)��n(O3)=3:2������O2��O3�ķ�����֮��Ϊ3:2��ԭ����֮��Ϊ��![]() =1:1�������Ϊ��3:2��1:1��3:2��
=1:1���������3:2��1:1��3:2��
��2�����µ�ѹ�£��������O2��O3����n(O2)/n(O3)=V(O2)/V(O3)=1:1�����Ӹ�����Ϊ1:1��ԭ�Ӹ�����Ϊ��2 n(O2)/3n(O3)=2:3������֮��Ϊ��32:48=2:3�������Ϊ��1:1��2:3��2:3��
��3����cg������x�������ӣ����У�a��b=c��x��x=![]() ��cg���������ʵ���Ϊ��n=
��cg���������ʵ���Ϊ��n=![]() mol������cg���������Ϊ��22.4L/mol��
mol������cg�����������22.4L/mol��![]() mol=
mol=![]() L���������
L���������![]() L��
L��
��4������ʱӦѡ1000mL����ƿ������n(NaOH)=1L![]() 2mol��L-1=2mol������Ϊ��2mol
2mol��L-1=2mol��������2mol![]() 40g/mol=80g���������80g��
40g/mol=80g���������80g��
��5����״���£�11.2L�����������ʵ���Ϊ��n=![]() =0.5mol������ƽ����������Ϊ��M=
=0.5mol������ƽ����������Ϊ��M=![]() =30g/mol������������CO��CO2�����ʵ����ֱ�Ϊx��y���õ�28x+44y=15��x+y=0.5����ã�x=
=30g/mol������������CO��CO2�����ʵ����ֱ�Ϊx��y���õ�28x+44y=15��x+y=0.5����ã�x=![]() �� y=
�� y=![]() ������CO2��CO�����֮����7:1�������Ϊ 30g/mol��7:1��
������CO2��CO�����֮����7:1�������Ϊ 30g/mol��7:1��

����Ŀ����֪��ӦA(g)��B(g) ![]() C(g)��D(g)��ƽ�ⳣ��Kֵ���¶ȵĹ�ϵ�����ʾ��830 ��ʱ����һ��2 L���ܱ������г���0.20 mol A��0.20 mol B,10 sʱ��ƽ�⡣����˵������ȷ����
C(g)��D(g)��ƽ�ⳣ��Kֵ���¶ȵĹ�ϵ�����ʾ��830 ��ʱ����һ��2 L���ܱ������г���0.20 mol A��0.20 mol B,10 sʱ��ƽ�⡣����˵������ȷ����
�¶�/�� | 700 | 830 | 1200 |
Kֵ | 1.7 | 1.0 | 0.4 |
A. �ﵽƽ���B��ת����Ϊ50%
B. ����ѹǿ�������淴Ӧ���ʾ��ӿ�
C. �÷�ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�������ƶ�
D. ��Ӧ��ʼ��ƽ�⣬A��ƽ����Ӧ����v(A)��0.005 mol��L��1��s��1