��Ŀ����
��14�֣���ҵ������CO������������֪��25��ʱ��
C(s,ʯī) + 1/2 O2(g) = CO(g) ��H1= -111kJ/mol
H2(g) + 1/2 O2(g) = H2O(g) ��H2= -242kJ/mol
C(s,ʯī) + O2(g) = CO2(g) ��H3= -394kJ/mol
��1����25��ʱ��CO(g)
+ H2O(g)  CO2(g) + H2(g) ��H=_____________��
CO2(g) + H2(g) ��H=_____________��
��2����2L�ܱ������У���2
mol CO��3 mol H2O��ϼ��ȵ�800�棬�������з�Ӧ��CO(g)+H2O(g)  CO2(g)+H2(g) K=1.0����ƽ���CO��ת����Ϊ_______��ƽ��������H2���������Ϊ_______��
CO2(g)+H2(g) K=1.0����ƽ���CO��ת����Ϊ_______��ƽ��������H2���������Ϊ_______��
��3������2���е�ƽ���Ļ������ͨ��300mL 6mol/L NaOH��Һ�У�������գ�������Һ������Ũ���ɴ�С��˳��Ϊ_________________________________________________��
��4������3����ʣ�������ͨ������Ũ������������������õ����ȼ��ͨ�������Ĺ��������У��������Ƶ���������______g��
��5����ҵ��Ҳ������CO��H2�����״���CO(g) + 2H2 (g) = CH3OH (g)����һ�������£��÷�Ӧ��һ���ܱ������дﵽƽ�⣬��ά��c(H2)���������¶Ȳ��䣬�����������������ƽ��_________������ĸ��
A�����ƶ� B��������Ӧ�����ƶ�
C�����淴Ӧ�����ƶ� D�����ж��ƶ��ķ���
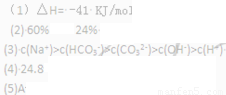
��������



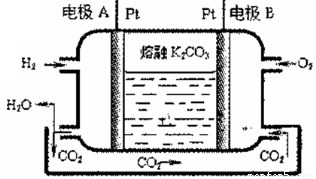
 CO2(g) + H2(g) ��H=_____________��
CO2(g) + H2(g) ��H=_____________�� _____________��
_____________�� (g) = CH3OH (g)����һ�������£��÷�Ӧ��һ���ܱ������дﵽƽ�⣬��ά��c(H2)���������¶Ȳ��䣬
(g) = CH3OH (g)����һ�������£��÷�Ӧ��һ���ܱ������дﵽƽ�⣬��ά��c(H2)���������¶Ȳ��䣬 �����������������ƽ��_________������ĸ��
�����������������ƽ��_________������ĸ��