��Ŀ����
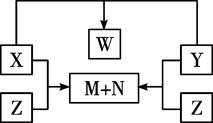
��15�֣���X��Y��Z��W���ֶ�����Ԫ��ԭ������������������֮�����ͬʱ��γ�A2B2�͡�AB�͡�A2�͡�A22���͵Ⱦ�����ͬ������������
��1����A2����Ϊ��������Ҫ�ɷ�ʱ��
��д������A22����������ʽ _______________��
��д������Ԫ��ԭ�Ӱ�5��1��1��3�γɵ����ӻ�����������NaOH��Ӧ�����ӷ���ʽ_______________��
��2����A2����Ϊ����õĵ���ʱ��
��1mol A2B2�ͻ�����ֽ�ת�Ƶ��ӵ����ʵ���Ϊ_______��
����д��һ�־�����ͬ���ӵ������Ǽ��Է��ӵ��л��ﻯѧʽ__________
�۱Ƚ�Z��W����Ԫ�ص��⻯��ķе���ˮ��Һ�����ԡ��е�________________����______________��
��1����A2����Ϊ��������Ҫ�ɷ�ʱ��
��д������A22����������ʽ _______________��
��д������Ԫ��ԭ�Ӱ�5��1��1��3�γɵ����ӻ�����������NaOH��Ӧ�����ӷ���ʽ_______________��
��2����A2����Ϊ����õĵ���ʱ��
��1mol A2B2�ͻ�����ֽ�ת�Ƶ��ӵ����ʵ���Ϊ_______��
����д��һ�־�����ͬ���ӵ������Ǽ��Է��ӵ��л��ﻯѧʽ__________
�۱Ƚ�Z��W����Ԫ�ص��⻯��ķе���ˮ��Һ�����ԡ��е�________________����______________��
��1��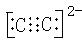 NH4++HCO3��+2OH��=NH3��H2O+CO32��+H2O
NH4++HCO3��+2OH��=NH3��H2O+CO32��+H2O
��2��1mol��3�֣� �� C2H6 ��3�֣� ���е㣺HF��HCl ����HCl��HF
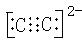 NH4++HCO3��+2OH��=NH3��H2O+CO32��+H2O
NH4++HCO3��+2OH��=NH3��H2O+CO32��+H2O��2��1mol��3�֣� �� C2H6 ��3�֣� ���е㣺HF��HCl ����HCl��HF
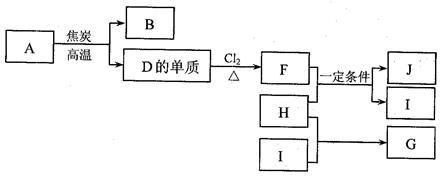
�����Ԫ���ƶ��Ե�����Ϊ�������ص���Ԫ�ػ�����֪ʶΪѧ�����ۺϵ����壬��ʵ��������������ۡ���ѧ���㡣�ԡ�A2���ͷ���Ϊͻ�ƿڡ��������ɶ�����Ԫ���γɵ�A2�ͷ�����5�֣�H2��N2��O2��F2��Cl2Ȼ����б任�Ƶ��������A2����Ϊ��������Ҫ�ɷ�ʱ���ɼ��������Ϊ14����ôA2�ͷ���ΪN2��AB�ͷ���ΪCO��A2B2�ͷ���ΪC2H2��������N�൱��CH������Ϊ�Ʊ���Ȳ�����ӻ�����̼�����д���C22������Ҳ��14���ӣ�����X��Y��Z��W���ֶ�����Ԫ������ΪH��C��N��O����Ϊ16���ӣ��Ҳ���A2B2������A2����Ϊ����õĵ���ʱ����ôA2�ͷ���ΪF2������Щ���ĵ�����Ϊ18��A2B2�ͷ���ΪH2O2��������F�൱��OH����O22��Ҳ�Ǻ���18���ӣ�AB�ͷ���ΪHCl��X��Y��Z��W���ֶ�����Ԫ������ΪH��O��F��Cl��

��ϰ��ϵ�д�
�����Ŀ
 ����������������������������������������
����������������������������������������